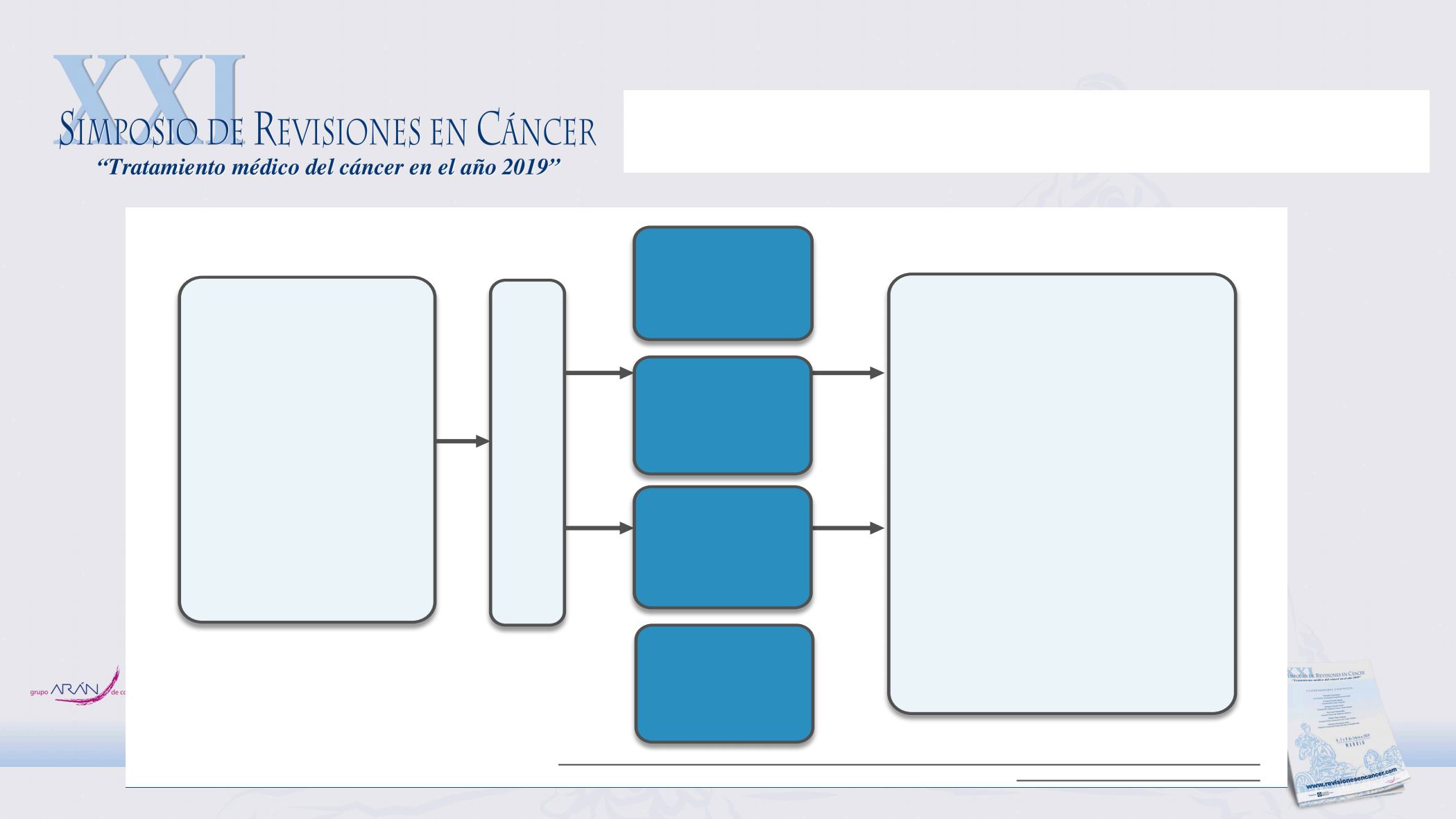
PEACE-1: Phase III of ADT + docetaxel +/- local
RT +/- AA in mHNPC
Clinicaltrials.gov: NCT01957436; Study synopsis:
http://www.urofrance.org/fileadmin/medias/recherche/protocoles/getug-21/synopsis-getug-21_2013-06-18_v2.pdf
R
A
N
D
O
M
I
Z
E
D
1:1:
1:1
Arm A: ADT
+ Doc
Co-primary endpoints
•
OS
•
PFS
Secondary endpoints
•
PSA response rate
•
PSA response/progression
•
rPFS
•
PC-specific survival
•
Time to pain progression
•
Time to next SRE
•
Time to chemotherapy
•
Time to grade 3/4 events
•
Impact of RT on PFS and
local symptoms
•
Toxicity
Patients with
newly diagnosed
(hormone-naïve)
metastatic
prostate cancer
Estimated
N = 1168
Arm B: ADT
+ Doc + AA
+ P
Arm A + RT
Arm B + RT
Estimated LPLV 2025
First interim analysis Q3 2019
EMEA Medical Affairs sponsored IIS
PEACE-1: Phase III of ADT + docetaxel +/- local
RT +/- AA in mHNPC
Clinicaltrials.gov: NCT01957436; Study synopsis:
http://www.urofrance.org/fileadmin/medias/recherche/protocoles/getug-21/synopsis-getug-21_2013-06-18_v2.pdf
R
A
N
D
O
M
I
Z
E
D
1:1:
1:1
Arm A: ADT
+ Doc
Co-primary endpoints
•
OS
•
PFS
Secondary endpoints
•
PSA response rate
•
PSA response/progression
•
rPFS
•
PC-specific survival
•
Time to pain progression
•
Time to next SRE
•
Time to chemotherapy
•
Time to grade 3/4 events
•
Impact of RT on PFS and
local symptoms
•
Toxicity
Patients with
newly diagnosed
(hormone-naïve)
metastatic
prostate cancer
Estimated
N = 1168
Arm B: ADT
+ Doc + AA
+ P
Arm A + RT
Arm B + RT
Estimated LPLV 2025
First interim analysis Q3 2019
EMEA Medical Affairs sponsored IIS








