Platinum Priority
–
Review
–
Prostate Cancer
Editorial by Rahul Aggarwal on pp. 845
–
846 of this issue
Comparison of Abiraterone Acetate and Docetaxel with Androgen
Deprivation Therapy in High-risk and Metastatic Hormone-naïve
Prostate Cancer: A Systematic Review and Network Meta-analysis
Christopher J.D. Wallis
a
,
y
,
*
, Zachary Klaassen
a
,
b
,
y
, Bimal Bhindi
c
, Hanan Goldberg
a
,
b
,
Thenappan Chandrasekar
a
,
b
, Ann M. Farrell
d
, Stephen A. Boorjian
c
, Girish S. Kulkarni
a
,
b
,
Robert Jeffrey Karnes
c
, Raj Satkunasivam
a
,
e
a
Division of Urology, Department of Surgery, University of Toronto, Toronto, ON, Canada;
b
Division of Urology, Department of Surgery, Princess Margaret
Hospital, University Health Network, Toronto, ON, Canada;
c
Department of Urology, Mayo Clinic, Rochester, MN, USA;
d
Mayo Clinic Libraries, Mayo Clinic,
Rochester, MN, USA;
e
Department of Urology and Center for Outcomes Research, Houston Methodist Hospital, Houston, TX, USA
Article info
Article history:
Accepted October 2, 2017
Associate Editor:
Giacomo Novara
Statistical Editor:
Melissa Assel
Keywords:
Metastatic prostate cancer
Locally advanced prostate cancer
Hormone-naïve
Docetaxel
Abiraterone
Androgen deprivation therapy
Abstract
Context:
Randomized clinical trials have recently examined the benefit of adding
docetaxel or abiraterone to androgen deprivation therapy (ADT) in hormone-naïve
advanced prostate cancer (PCa).
Objective:
To perform a systematic review and network meta-analysis of randomized
clinical trials, indirectly evaluating overall survival (OS) for men treatedwith abiraterone
acetate plus prednisone/prednisolone with ADT (Abi-ADT) versus docetaxel with ADT
(Doce-ADT) in hormone-naïve high-risk and metastatic PCa.
Evidence acquisition:
Medline, Embase, Web of Science, Scopus, and Clinicaltrials.gov
databases were searched in August 2017. We pooled results using the inverse variance
technique and random-effects models. The Bucher technique for indirect treatment
comparison was used to compare Abi-ADT with Doce-ADT. A priori subgroup and
sensitivity analyses were performed.
Evidence synthesis:
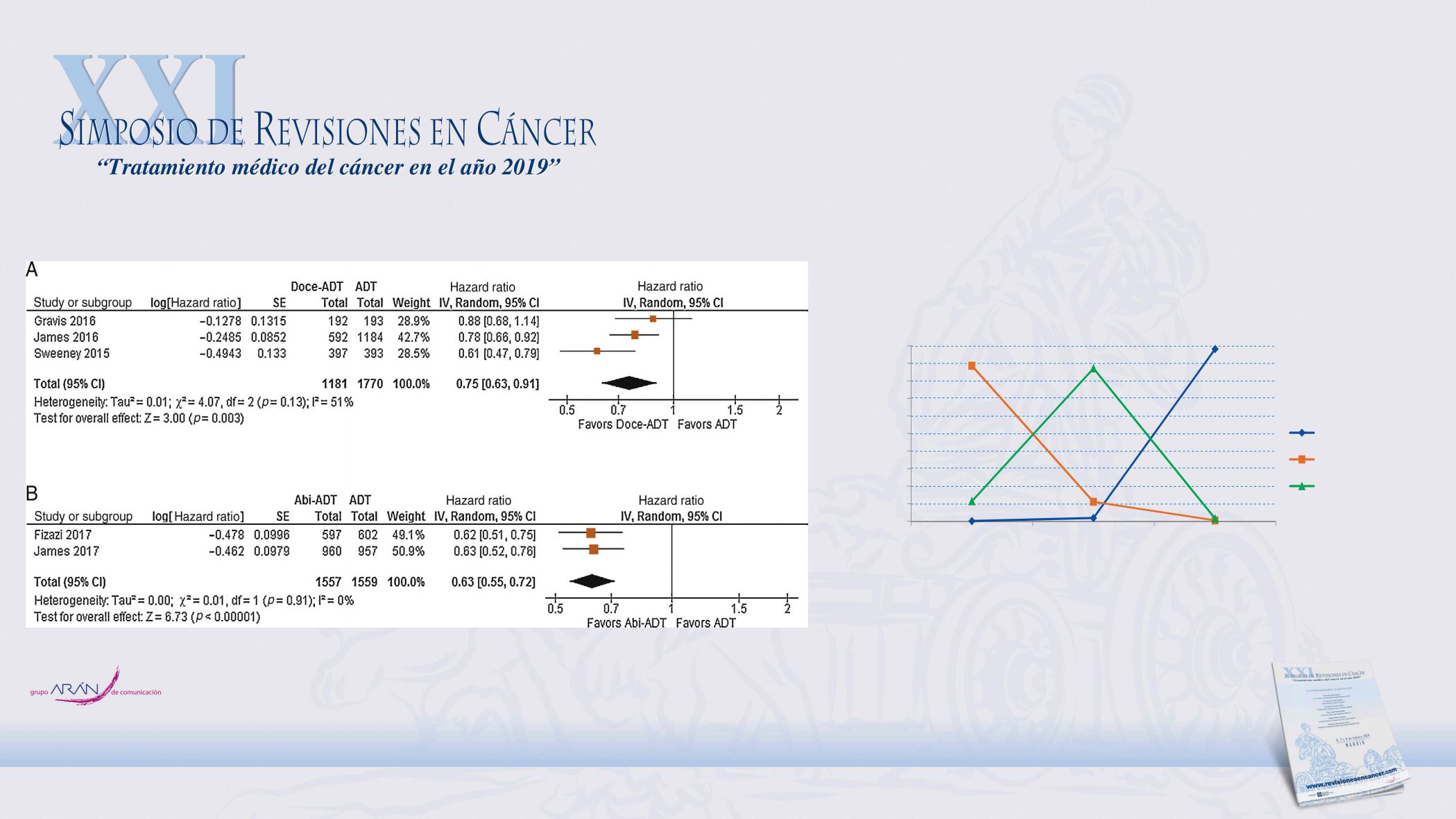
Overall, 6067 patients from
fi
ve trials were included: 1181 (19.5%)
patients who received Doce-ADT, 1557 (25.7%) patients who received Abi-ADT, and
3329 (54.9%) patients who received ADT-alone. There was a total of 1921 deaths:
391 in the Doce-ADT group, 353 in the Abi-ADT group, and 1177 in the ADT-only
group. The pooled hazard ratio (HR) for OS was 0.75 (95% con
fi
dence interval [CI]:
0.63
–
0.91, I
2
= 51%, 3 trials, 2951 patients) for Doce-ADT versus ADT-alone and 0.63
(95% CI: 0.55
–
0.72, I
2
= 0%, 2 trials, 3116 patients) for Abi-ADT versus ADT-alone.
The indirect comparison of Abi-ADT to Doce-ADT demonstrated no statistically
signi
fi
cant difference in OS between these approaches (HR: 0.84, 95% CI: 0.67
–
1.06). Findings were similar in various a priori subset analyses, including patients
with metastatic disease. Bayesian analyses demonstrated comparable results (HR:
0.83, 95% CI: 0.63
–
1.16). Despite the lack of statistical signi
fi
cance, Surface Under the
Cumulative Ranking Analysis demonstrated an 89% probability that Abi-ADT was
preferred.
Wallis et al. Eur Urol 2018
g. 1
–
Forest plot for meta-analysis of combination therapy compared to androgen deprivation therapy (ADT) alone: (A) docetaxel plus ADT versus
T alone and (B) abiraterone plus ADT versus ADT alone.
i = abiraterone acetate; CI = confidence interval; df = degrees of freedom; Doce = docetaxel; HR = hazard ratio; IV = instrumental variables;
= standard error.
0.7
0.8
0.9
1
bility
E U R O P E A N U R O L O GY 7 3 ( 2 0 18 ) 8 3 4
–
8 4 4
841
Abi-ADT versus Doce-ADT demonstrated no statistically
significant difference in OS between th s approaches (HR:
0.84, 95% CI: 0.67
–
1.06; Supplementary Fig. 2). Network
comparable results (HR: 0.83, 95
the lack of statistical significan
showed that Abi-ADT had an 89%
Fig. 1
–
Forest plot for meta-analysis of combination therapy compared to androgen deprivation therapy (ADT) alone: (A)
ADT alone and (B) abi aterone plu ADT versus ADT alone.
Abi = abiraterone acetate; CI = confidence interval; df = degrees of fre dom; D ce = docetaxel; HR = hazard ratio; IV = instru
SE = standard ror.
[(Fig._2)TD$FIG]
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
3rd
2nd
1st
Probability
Ranking strategies
ADT
ADT_Abi
ADT_Doce
Fig. 2
–
Surface Under the Cumulative Ranking plot demonstrating the probabilities of the rank order for each treatment
Abi = abiraterone acetate; ADT = androgen dep ivation therapy; Doce = docetaxel.








