Davendra P.S. Sohal, Erin B. Kennedy, Alok Khorana, Mehmet S. Copur, Christopher H. Crane, Ignacio Garrido-Laguna,
Smitha Krishnamurthi, Cassadie Moravek, Eileen M. O
’
Reilly, Philip A. Philip, Ramesh K. Ramanathan,
Joseph T. Ruggiero, Manish A. Shah, Susan Urba, Hope E. Uronis, Michelle W. Lau, and Daniel Laheru
A B S T R A C T
Purpose
In 2016, ASCO published a guideline to assist in clinical decision making in metastatic pancreatic
cancer for initial assessment after diagnosis,
fi
rst- and second-line treatment options, palliative and
supportive care, and follow-up. The purpose of this update is to incorporate new evidence related to
second-line therapy for patients who have experienced disease progression or intolerable toxicity
during
fi
rst-line therapy.
Methods
ASCO convened an Expert Panel to conduct a systematic review of the literature on second-line
therapy published between June 2015 and January 2018. Recommendations on other topics
covered in the 2016 Metastatic Pancreatic Cancer Guideline were endorsed by the Expert Panel.
Results
Two new studies were found that met the inclusion criteria.
Recommendations
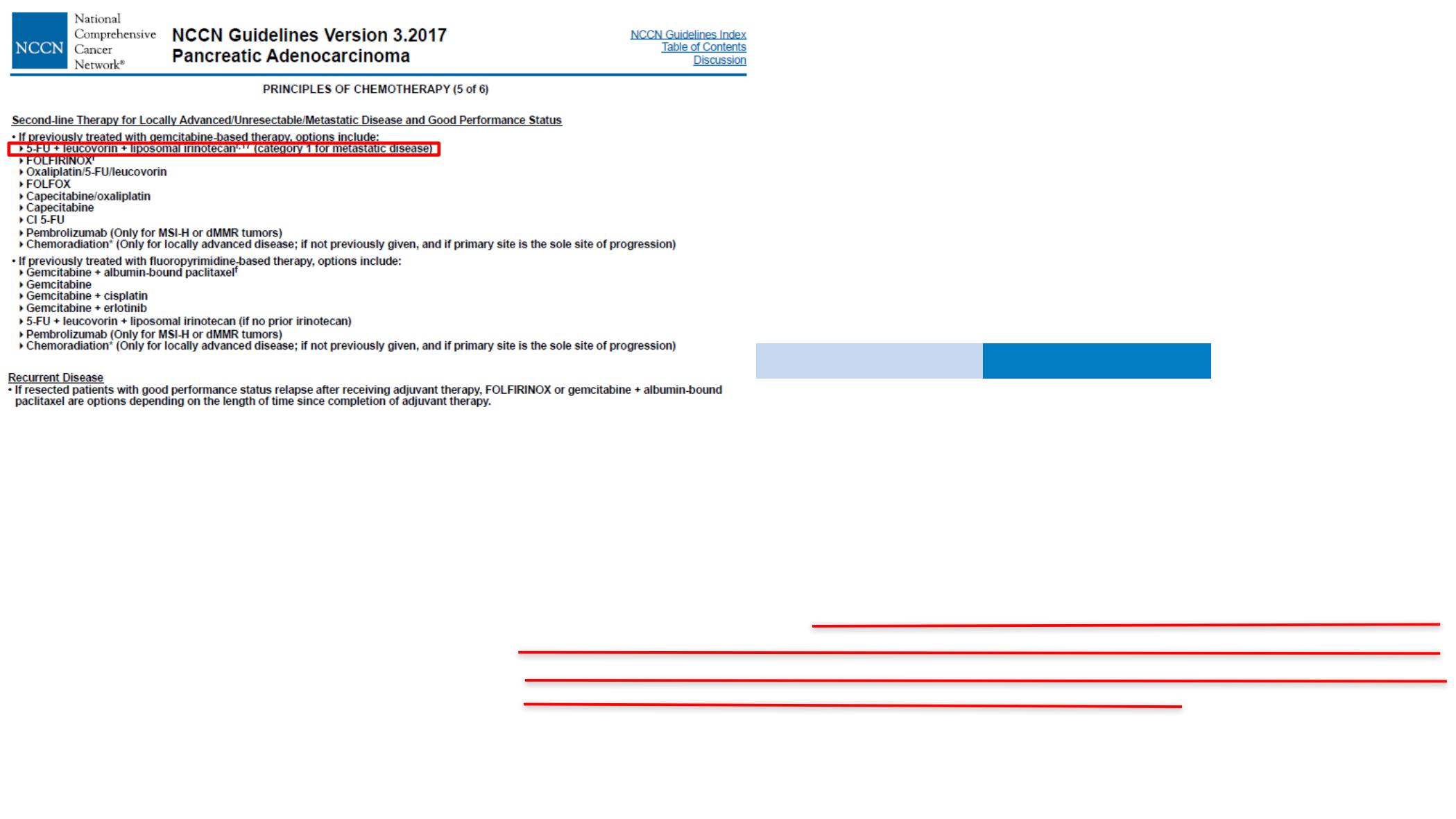
For second-line therapy, gemcitabine plus nanoparticle albumin-bound paclitaxel should be offered
to patients with
fi
rst-line treatment with FOLFIRINOX (leucovorin,
fl
uorouracil, irinotecan, and
oxaliplatin), an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 1, and
a favorable comorbidity pro
fi
le;
fl
uorouracil plus nanoliposomal irinotecan can be offered to patients
with
fi
rst-line treatment with gemcitabine plus NAB-paclitaxel, an ECOG PS of 0 to 1, and a favorable
comorbidity pro
fi
le;
fl
uorouracil plus irinotecan or
fl
uorouracil plus oxaliplatin may be offered when
there is a lack of availability of
fl
uorouracil plus nanoliposomal irinotecan; gemcitabine or
fl
uorouracil
should be offered to patients with either an ECOG PS of 2 or a comorbidity pro
fi
le that precludes
other regimens. Testing select patients for mismatch repair de
fi
ciency or microsatellite instability is
recommended, and pembrolizumab is recommended for patients with mismatch repair de
fi
ciency
or high microsatellite instability tumors. Endorsed recommendations from the 2016 version of this
Author af
fi
liations and support information
(if applicable) appear at the end of this
article.
Published at
jco.orgon May 23, 2018.
D.P.S.S. and D.L. were Expert Panel
co-chairs.
Clinical Practice Guideline Committee
approved: March 8, 2018.
Editor
’
s note: This American Society of
Clinical Oncology (ASCO) Clinical Practice
Guideline provides recommendations,
with comprehensive review and analyses
of the relevant literature for each
recommendation. Additional information,
including a Data Supplement,
a Methodology Supplement, slide sets,
clinical tools and resources, and links to
patient information at
www.cancer.net ,is
available at
www.asco.org/gastrointestinal-cancer-guidelines
.
Authors
’
disclosures of potential con
fl
icts
of interest and author contributions are
found at the end of this article.
Reprint requests: 2318 Mill Road, Suite
800, Alexandria, VA 22314; guidelines@
asco.org.
Corresponding author: American Society
of Clinical Oncology, 2318 Mill Rd, Suite
800, Alexandria, VA 22314; e-mail:
guidelines@asco.org.
J
OURNAL OF
C
LINICAL
O
NCOLOGY
A S C O S P E C I A L A R T I C L E
Met static Pancreatic Cancer: ASCO Clinical Practice
Guideline Update
DavendraP.S.Sohal,ErinB.Kennedy,AlokKhorana,MehmetS.Copur,ChristopherH.Crane,IgnacioGarrido-Laguna,
Smitha Krishnamurthi, Cassadie Moravek, Eileen M. O
’
Reilly, Philip A. Philip, Ramesh K. Ramanathan,
Joseph T. Ruggiero, Manish A. Shah, Susan Urba, Hope E. Uronis, Michelle W. Lau, and Daniel Laheru
A B S T R A C T
Purpose
In 2016, ASCO published a guideline to assist in clinical decision making in metastatic pancreatic
cancer for initial assessment after diagnosis,
fi
rst- and second-line treatment options, palliative and
supportive care, and foll w-up. The purpose of this update is to incorporate new evidence related to
second-line therapy for patients who have experienced disease progression or intolerable toxicity
during
fi
rst-line therapy.
Methods
ASCO convened an Expert Panel to conduct a systematic review of the literature on second-line
therapy published between June 2015 and January 2018. Recommendations on other topics
covered in the 2016 Metastatic Pancreatic Cancer Guideline were endorsed by the Expert Panel.
Results
Two new studies were found that met the inclusion criteria.
Recommendations
For second-line therapy, gemcitabine lus nanoparticle albumin-bou d paclitaxel should be offered
to patients with
fi
rst-line treatment with FOLFIRINOX (leucovorin,
fl
uorouracil, irinotecan, and
oxaliplatin), an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 1, and
a favorable comorbidity pro
fi
le;
fl
uorouracil plus nanoliposomal irinotecan can be offered to patients
with
fi
rst-line treatment with gemcitabine plus NAB-paclitaxel, an ECOG PS of 0 to 1, and a favorable
comorbidity pro
fi
le;
fl
uorouracil plus irinotecan or
fl
uorouracil plus oxaliplatin may be offered when
there is a lack of availability of
fl
uorouracil plus nanoliposomal irinotecan; gemcitabine or
fl
uorouracil
should be offered to patients with either an ECOG PS of 2 or a comorbidity pro
fi
le that precludes
other regimens. Testing select patients for mismatch repair de
fi
ciency or microsatellite instability is
recommended, and pembrolizumab is recommended for patients with mismatch repair de
fi
ciency
or high microsatellite instability tumors. Endorsed recommendations from the 2016 version of this
guideline for co puted tomography, baseline performance status and comorbidity pro
fi
le, de
fi
ning
goals of care,
fi
rst-line therapy, and palliative care are also contained within the full guideline text.
Additional information is available at
www.asco.org/gastrointestinal-cancer-guidelines.
J Clin Oncol 36. © 2018 by American Society of Clinical Oncology
cancer. The guideline provided recommendations
Authoraf
fi
liationsandsupportinformation
(if applicable) appear at the end of this
article.
Published at
jco.orgon May 23, 2018.
D.P.S.S. and D.L. were Expert Panel
co-chairs.
Clinical Practice Guideline Committee
approved: March 8, 2018.
Editor
’
s note: This American Soci ty of
ClinicalOncology(ASCO)ClinicalPractice
Guideline provides recommendations,
withcomprehensivereviewandanalyses
of the relevant literature for each
recommendation. Additional information,
including a Data Supplement,
a Methodology Supplement, slide sets,
clinical tools and resources, and links to
patientinformationat
www.cancer.net ,is
available at
www.asco.org/gastrointestinal-cancer-guidelines
.
Authors
’
disclosuresofpotentialcon
fl
icts
of interest and author contributions are
found at the end of this article.
Reprint requests: 2318 Mill Road, Suite
800, Alexandria, VA 22314; guidelines@
asco.org.Corresponding author: American Society
of Clinical Oncology, 2318 Mill Rd, Suite
800, Alexandria, VA 22314; e-mail:
guidelines@asco.org.
© 2018 by American Society of Clinical
Oncology
0732-183X/18/3699-1/$20.00
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines):
Pancreatic Adenocarcinoma v3.2017.
www.nccn.org/professionals/physician_gls/f_guidelines.asp.Accessed
24 September 2017.








