•
Study endpoints: OS (1°); PFS, time to treatment failure and ORR (2°); other
endpoints included CA19-9 (tumour marker) response and safety
•
Stratification factors: serum albumin levels, KPS, ethnicity
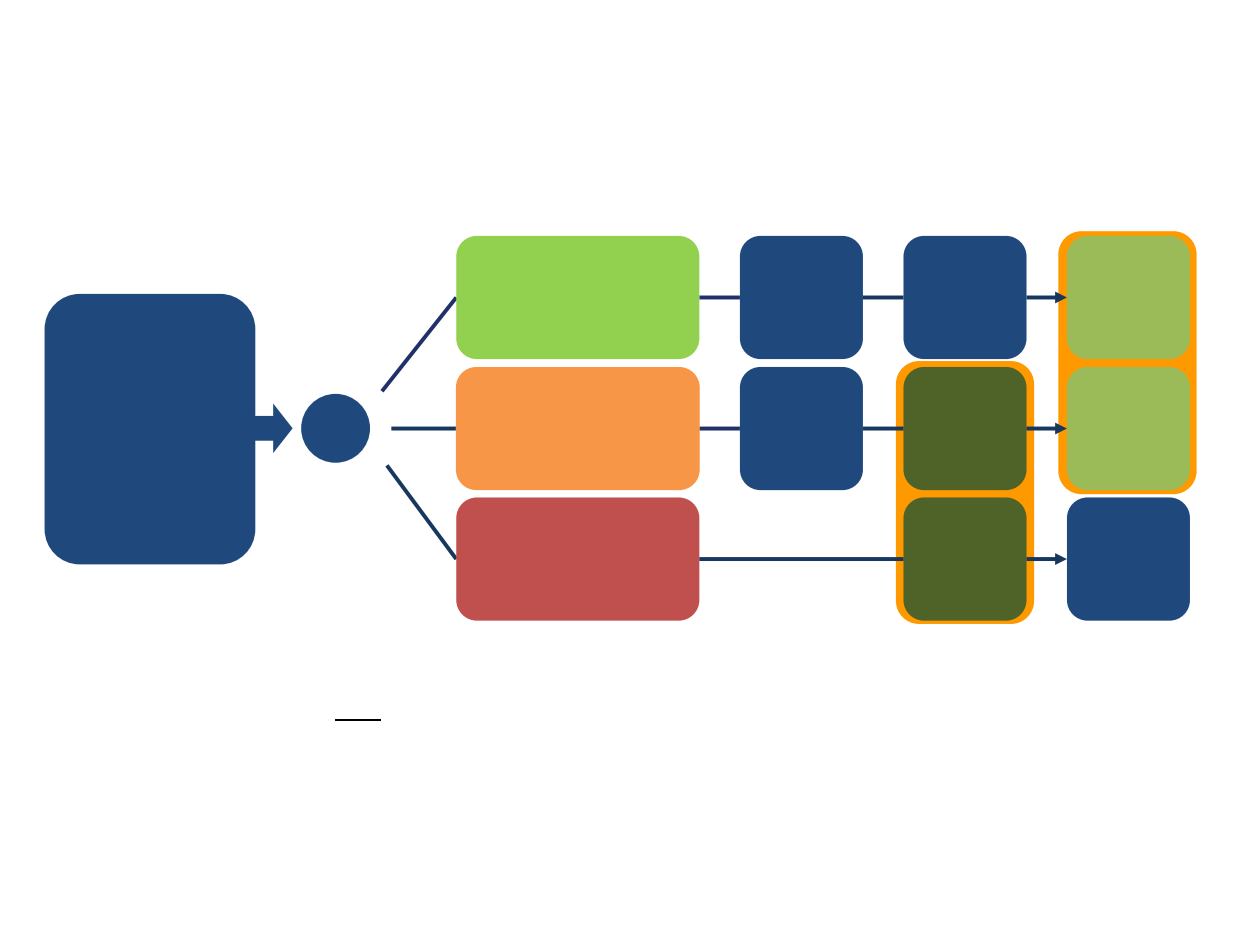
R
5-FU/LV
(2000/200 mg/m
2
QW x 4, Q6W)
NAPOLI:
nal-IRI+5-FU/LV
(80 mg/m
2
+ 2400/400
mg/m
2
Q2W)
Initial design
n = 33
n = 30
n = 118
n = 119
n = 117
n = 151
n = 149
n = 117
Total
nal-IRI
(120 mg/m² Q3W)
Post-
amendment
Patients with
metastatic
pancreatic
cancer that
progressed after
previous
gemcitabine-
based treatment
(n = 417)
NAPOLI-1: a randomised, open-label, global, multicentre,
phase III study of nal-IRI+5-FU/LV
Wang-Gillam A, et al. Lancet 2016;387:545








