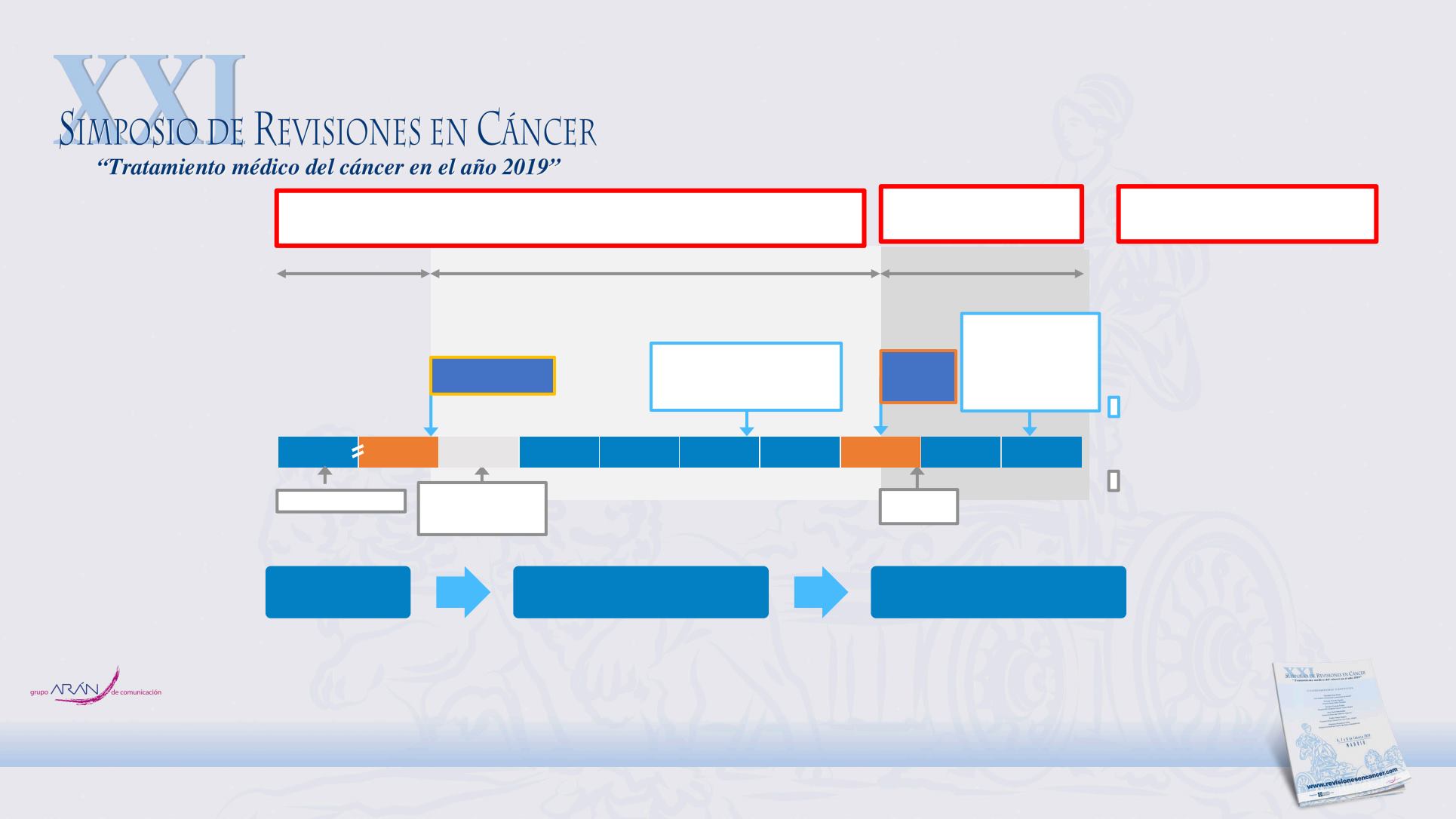
Biomarcadores
1.
http://ec.europa.eu/health/documents/community-register/html/h423.htm(accessed 08-03-18);
2.
http://ec.europa.eu/health/documents/community-register/html/h281.htm(accessed 08-03-18).
*This slide shows selected label updates, focusing on changes relating to the patient population eligible for anti-EGFR therapy. Therapeutic indications have been abbreviated; please
see product labels for full details;
†
and intolerant to irinotecan;
‡
for patients who have received 1
st
-line fluoropyrimidine-based chemotherapy (excluding irinotecan).
CTx, chemotherapy; CTxR, chemotherapy refractory; IRI, irinotecan.
2007
2004
Panitumumab
1
Cetuximab
2
EU label variations*
Combination: IRI (CTxR)
Monotherapy (CTxR)
(WT KRAS)
Combination:
FOLFOX or FOLFIRI (1
st
line);
FOLFIRI (2
nd
line
‡
);
monotherapy (CTxR)
(WT
RAS)
Combination: CTx;
Monotherapy (CTxR
†
)
(WT KRAS)
Label variation
(WT RAS)
Combination: FOLFOX (1
st
line);
FOLFIRI (2
nd
line);
monotherapy (CTxR)
(WT KRAS)
2008 2009 2010 2011 2012 2013 2014 2015
Unselected
WT KRAS exon 2
WT RAS
All
KRAS
RAS
Label
variation
(WT RAS)
PASADO
PRESENTE
FUTURO








