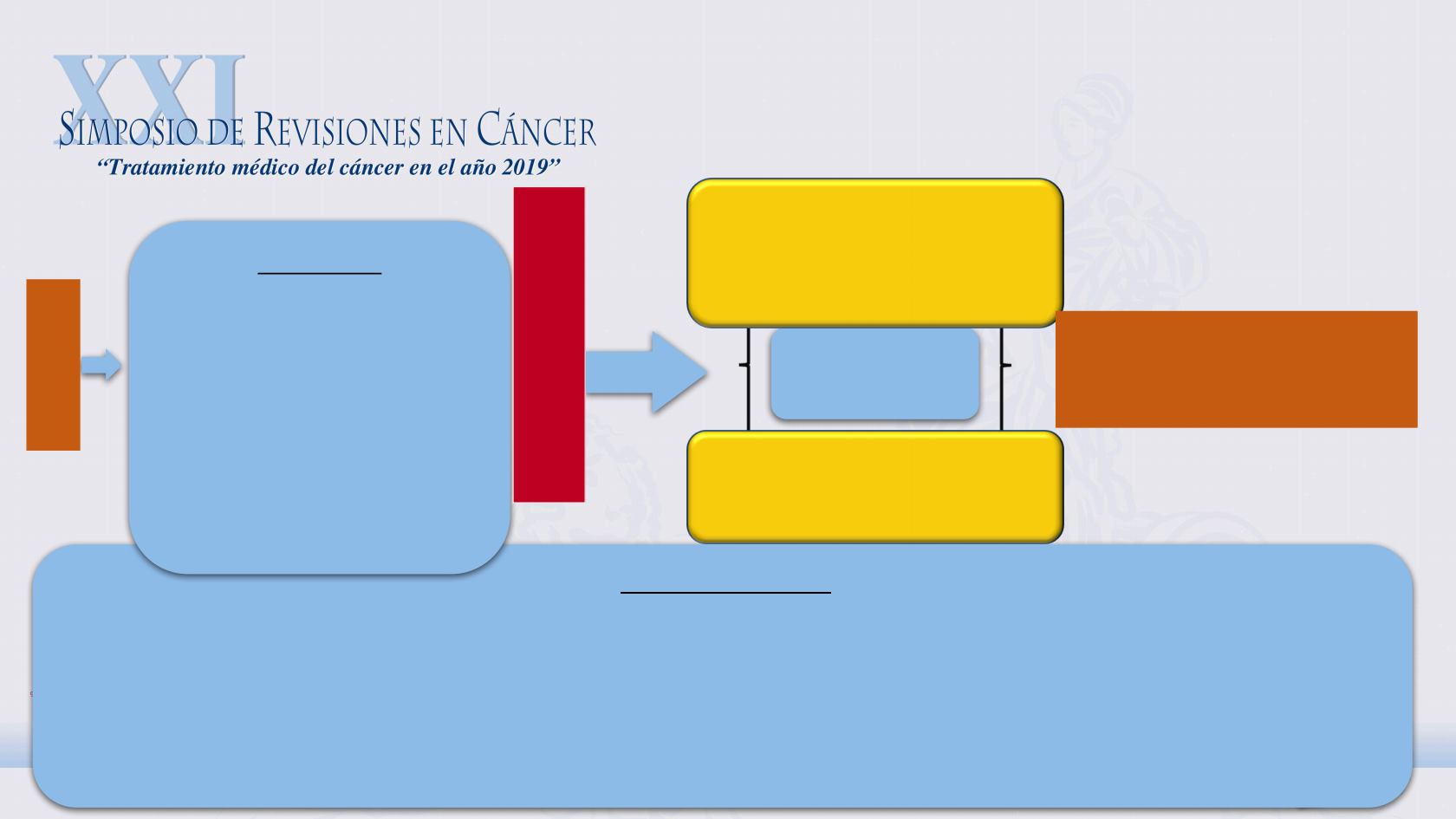
RAINBOW: Phase 3 Randomized Study in 2
nd
-line Gastric and GEJ Cancer, Ramucirumab with
Paclitaxel (CP12-0922/I4T-IE-JVBE)
Until
Progression
Patient Population
•
Gastric or GEJ adenocarcinoma (metastatic or locally advanced and unresectable)
•
1
st
-line therapy with platinum/fluoropyrimidine doublet, with or without anthracycline
•
Patient experienced progression during 1
st
-line therapy or within 4 months after 1
st
-line therapy
•
ECOG PS 0/1
•
No CNS metastases
Stratify by:
•
Time to progression on 1
st
-
line therapy
(< 6 vs ≥ 6 months)
•
Geographic region
•
Disease measurablility
(measurable vs.
nonmeasurable)
Ramucirumab 8 mg/kg every
2 weeks
+ Paclitaxel 80 mg/m
2
weekly
Placebo every 2 weeks
+ Paclitaxel 80 mg/m
2
weekly
N = 663
S
C
R
E
EN
R
A
N
D
O
M
I
Z
E
1:1
•
Tumor assessment every 6 weeks
•
Survival follow-up
•
Safety follow-u
p








