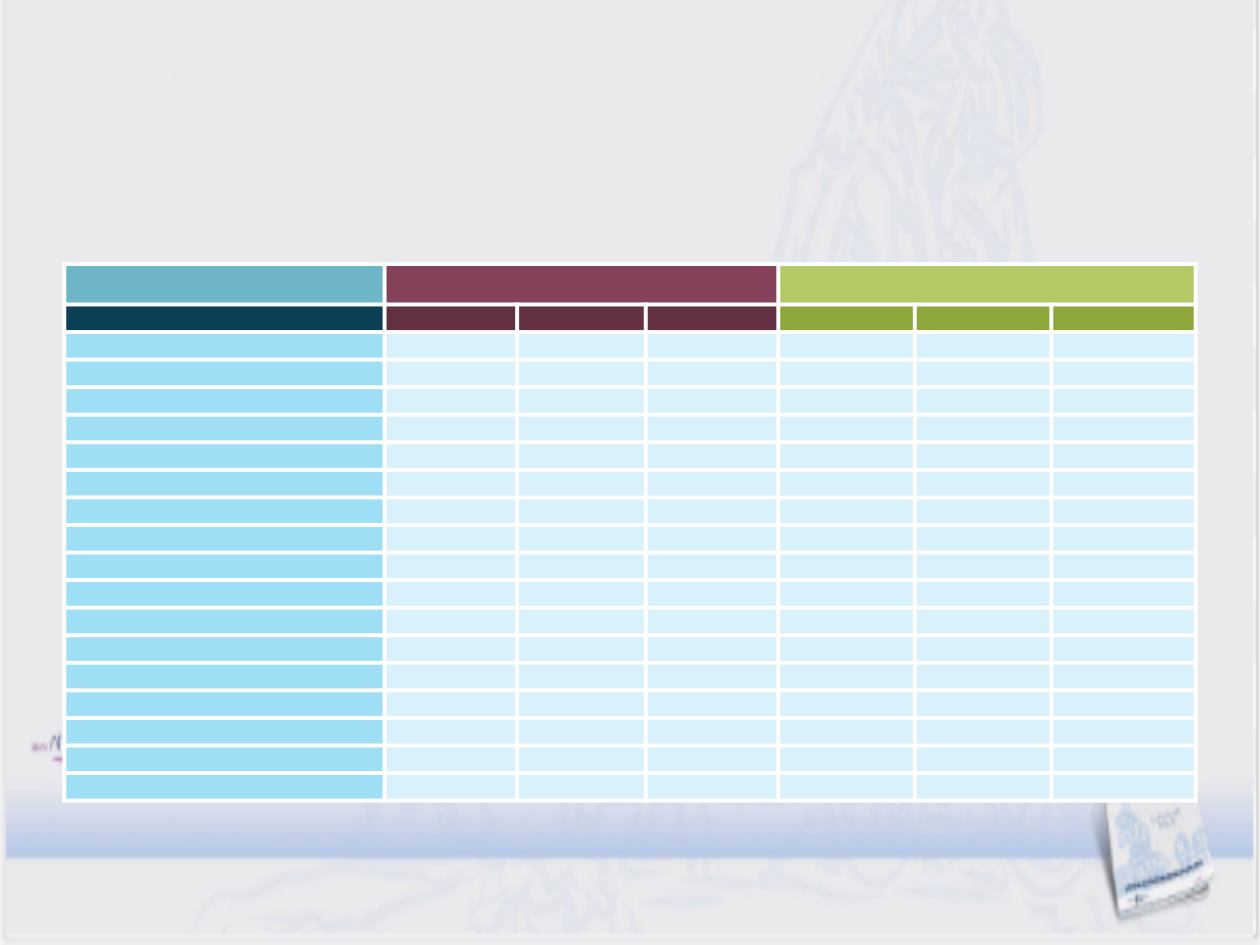
CABOSUN: Seguridad
Efectos adversos reportados G1/2 ≥20%
Cabozantinib (n=78)
Sunitinib (n=72)
Adverse event, %
Grade 1/2 Grade 3
Grade 4
Grade 1/2
Grade 3
Grade 4
Any AE
24
58
10
24
58
7
Diarrhoea
b
63
10
0
43
11
0
AST increased
b
58
1
1
28
3
0
Fatigue
b
58
6
0
51
17
0
ALT increased
b
50
4
1
28
0
0
Appetite decreased
42
5
0
31
1
0
Dysgeusia
41
0
0
29
0
0
Hypertension
b
39
28
0
24
19
1
Platelet count decreased
b
38
1
0
50
8
3
PPE syndrome
b
35
8
0
29
4
0
Anaemia
32
1
0
43
3
0
Stomatitis
32
5
0
24
6
0
Nausea
29
3
0
35
4
0
Weight decreased
28
4
0
17
0
0
Dyspepsia
27
0
0
17
0
0
Neutrophil count decreased
b
15
0
0
31
4
0
White blood cell count decreased
12
0
0
32
3
0
Choueiri TK, et al.
J Clin Oncol
2017;35:591–7 ("Errata."
J Clin Oncol,
35(32), p. 3736)








