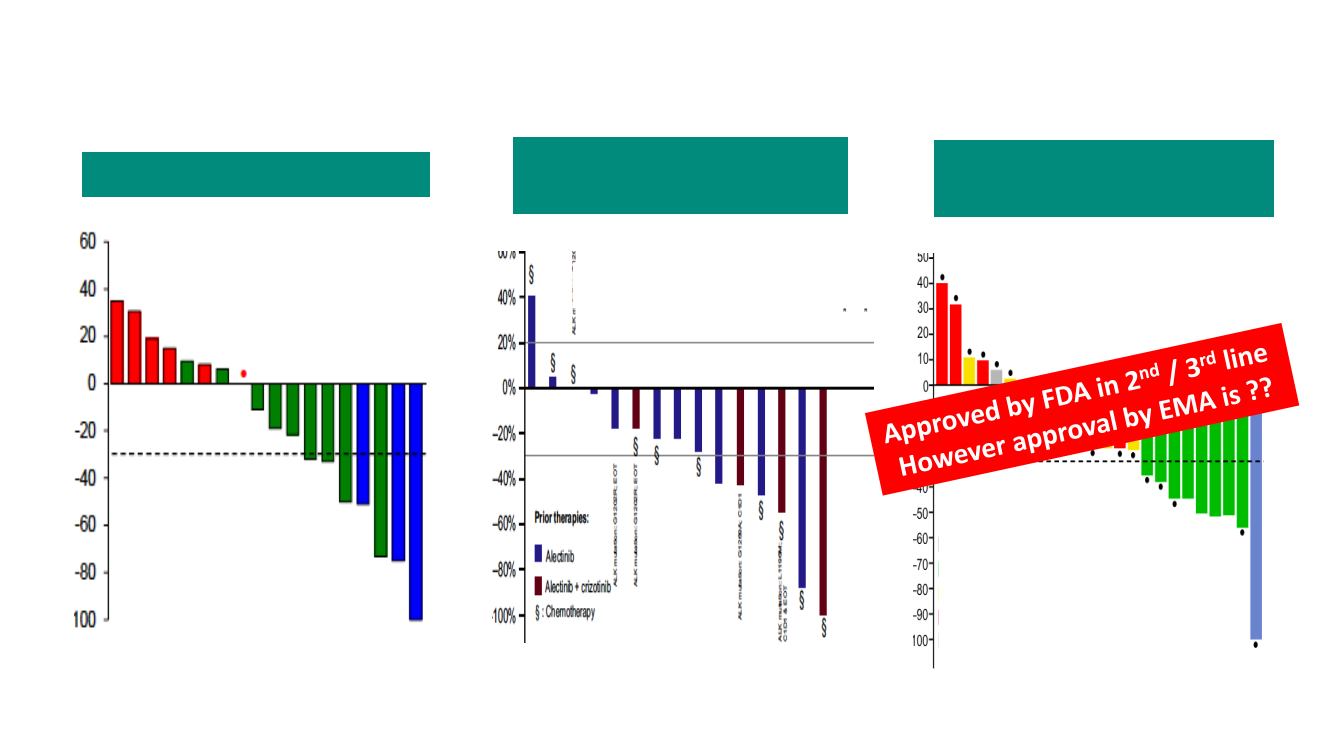
After 2
nd
generation ALK TKI…
Alectinib
à
Brigatinib (N=22)
Lin – JTO 2018
RR 17%. PFS 4.4 mo.
Alectinib
à
Ceritinib (N=20)
ASCEND-9 phase II Trial
Hida- Cancer Sci 2018
17 patients ≥2 ALK TKI
No efficacy in
G1202R
Post brigatinib, lorlatinib
RR 20%. PFS 3.7 mo.
Solomon – Lancer Oncol 2018 * Besse – ASCO 2018
Lorlatinib post-2
ND
G ALK TKI
+/- CT (EXP 3B. N=28)
Post
RR 32%. PFS 5.5 mo.
icRR: 55%
Post-Alectinib: 40%. 5.5 mo.
Post-Ceritinib: 43%. 7.3 mo.
Post-Brigatinib: 38%. NC








