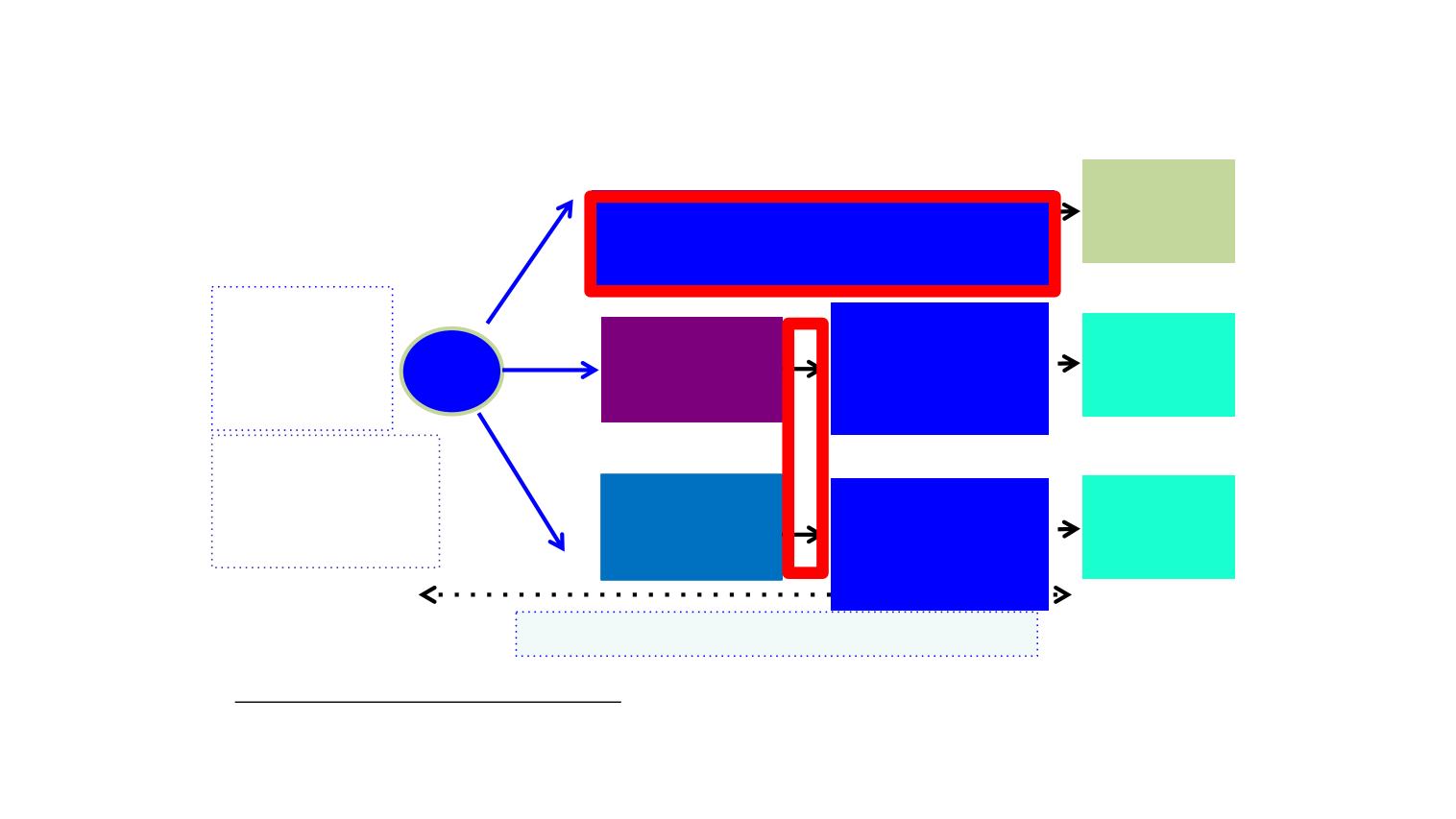
APPLE: Trial Design
Randomized, open-label, multicenter, phase II trial
Advanced NSCLC
Common
mEGFR
Treatment naïve
PS 0-2
Stable BM
Gefitinib*
Until cfDNA
PD (T790M+)
R
1:1:
1
Osimertinib until
RECIST PD
Rebiopsy
At PD by
RECIST
(optional)
ARM C
ARM A
Osimertinib
until
RECIST PD
Rebiopsy
At PD by
RECIST
(optional)
Primary End Point: PFS rate at 18 months
Gefitinib
Until RECIST
PD
Osimertinib
until
RECIST PD
Rebiopsy
At PD by
RECIST
(optional)
(cfDNA by COBAS every 4 weeks + CT-scan brain-thorax-abdominal / 8 weeks all arms
*In case of RECIST progression without T790M+, patients will be switched
ARM B
Stratification:
-Del19 vs. L858R
-
Initial T790M
+ / -
-BM + / -
N=156








