FLAURA—Front-line osimertinib for
EGFR
mutant NSCLC
CNS, central nervous system; EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; PFS, progression-free survival; p.o., orally; RECIST 1.1, Response Evaluation Criteria In Solid Tumors version 1.1; qd, once daily; SoC,
standard-of-care; TKI, tyrosine kinase inhibitor; WHO, World Health Organization
Stratification by
mutation
status
(Exon 19
deletion /
L858R)
and
race
(Asian /
non-Asian)
Crossover was allowed for patients
in the
SoC
arm, who could receive
open-label osimertinib upon central
confirmation of progression and
T790M positivity
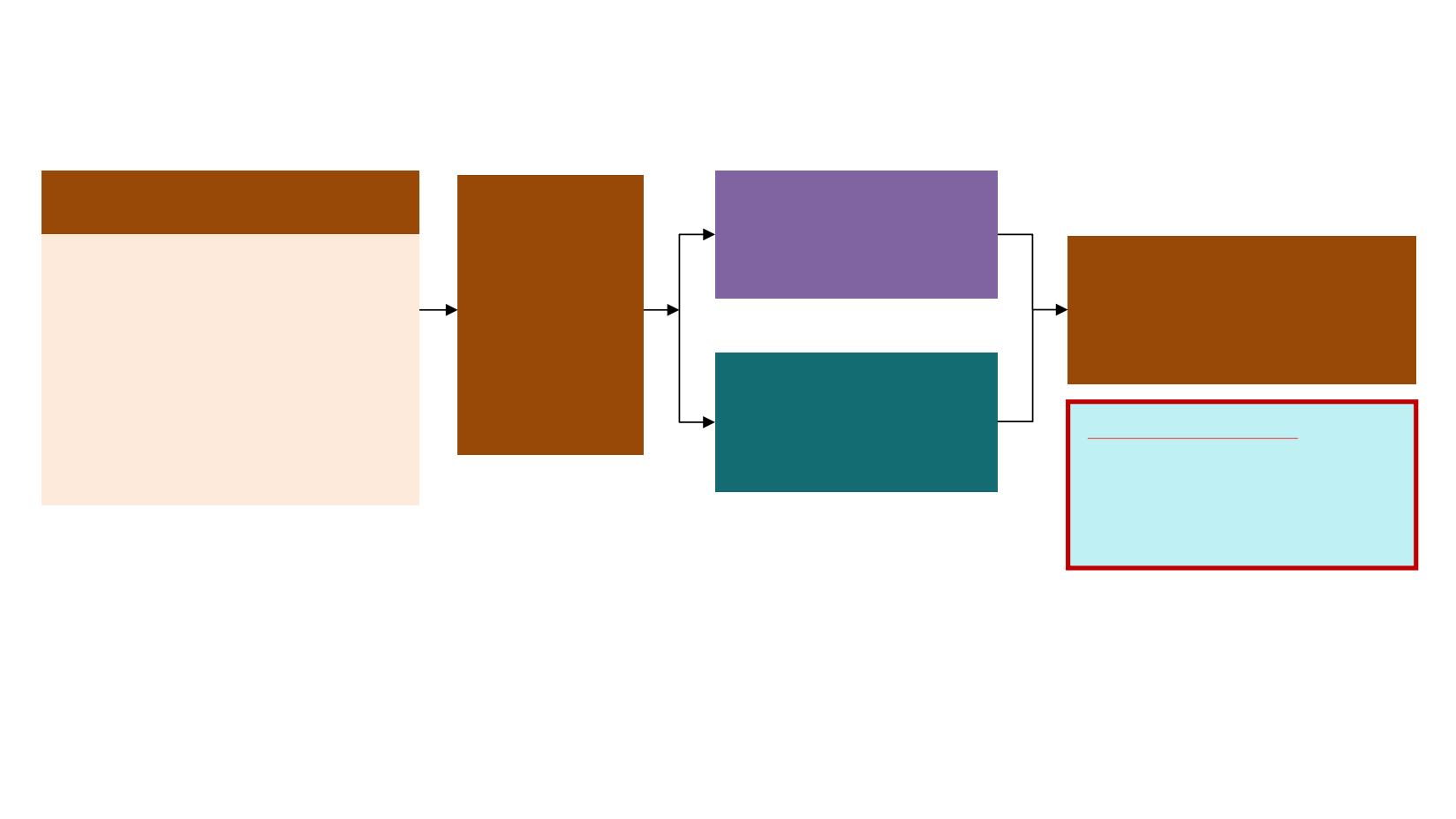
Patients with locally advanced or
metastatic NSCLC
Key inclusion criteria
•
≥18 years*
•
WHO performance status 0 / 1
•
Exon 19 deletion / L858R (enrolment
by local
#
or central
‡
EGFR testing)
•
No prior systemic anti-cancer /
EGFR-TKI therapy
•
Stable CNS metastases allowed
Endpoints
•
Primary endpoint:
PFS based on investigator assessment (according to RECIST 1.1)
•
The study had a 90% power to detect a hazard ratio of 0.71 (representing a 29% improvement in median PFS from
10 months to 14.1 months) at a two-sided alpha-level of 5%
•
Secondary endpoints:
objective response rate, duration of response, disease control rate, depth of
response, overall survival, patient reported outcomes, safety
Randomised 1:1
RECIST 1.1 assessment every
6 weeks
¶
until objective
progressive disease
EGFR-TKI SoC
§
;
Gefitinib
(250 mg p.o. qd) or
Erlotinib
(150 mg p.o. qd)
(n=277)
Osimertinib
(80 mg p.o. qd)
(n=279)








