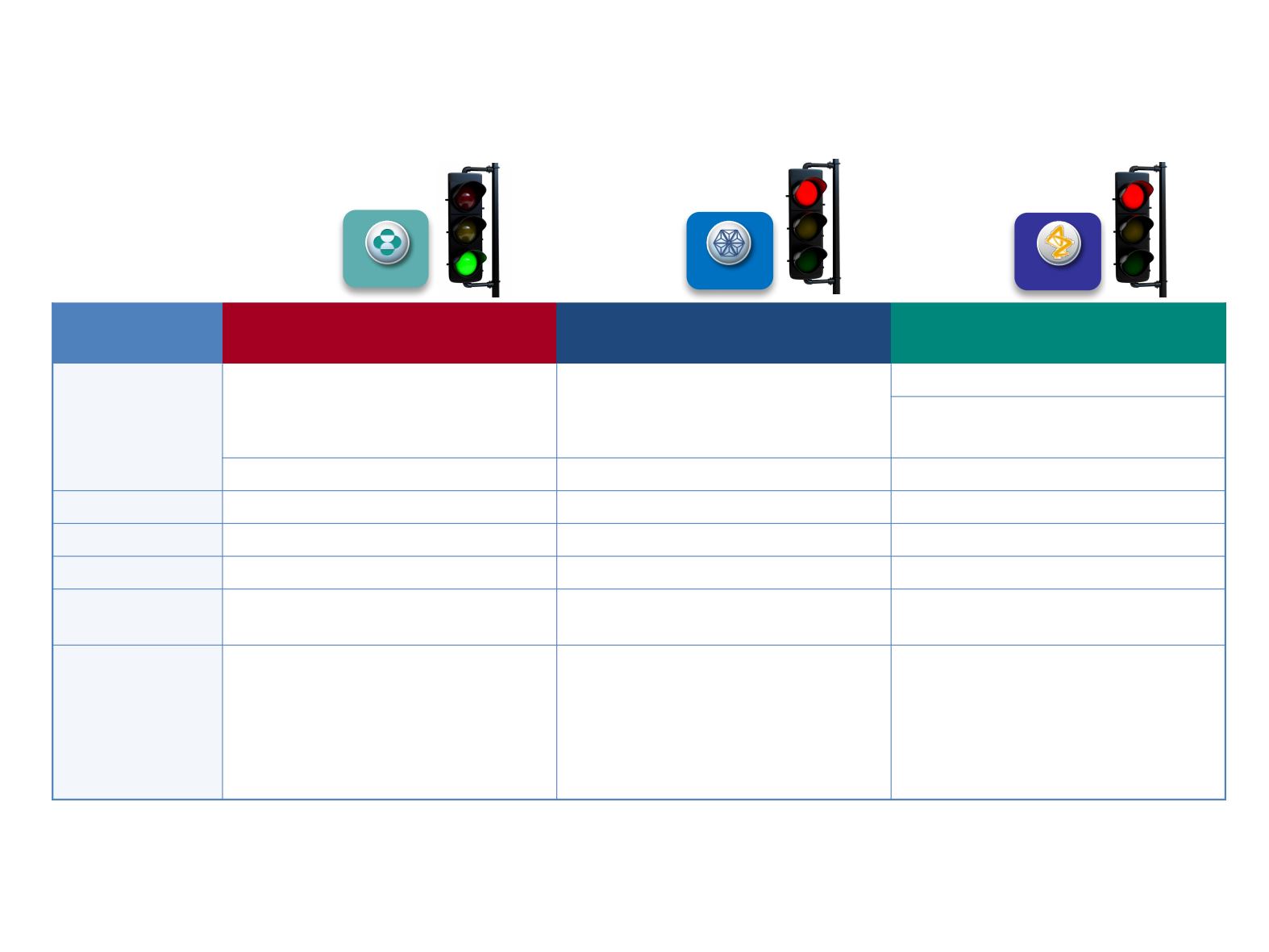
1L NSCLC: Phase 3 Trials With I-O Monotherapy
KEYNOTE-024
1
/
KEYNOTE-042
2
Checkmate 026
3
MYSTIC
4,5
Study Arms
Pembrolizumab 200 mg q3w
Nivolumab 3 mg/kg q2w
Durvalumab 20 mg/kg q4w
Durvalumab 20 mg/kg q4w
+ tremelimumab 1 mg/kg q4w
Pt-based chemo
Pt-based chemo
Pt-based chemo
N
305 / 1274
541
675
PD-L1 Cutoff
≥50% / ≥1%
≥5%*
≥25%
PD-L1 Assay
Dako 22C3
Dako 28-8
Ventana SP263
Primary
Endpoint(s)
PFS / OS
PFS
PFS, OS
Key Findings
•
PFS benefit demonstrated with
pembrolizumab in patients with
PD-L1 ≥50%
•
OS benefit demonstrated with
pembrolizumab in patients with
PD-L1 ≥1%
PFS benefit
not
demonstrated with
nivolumab in patients with
PD-L1
≥5%
PFS and OS benefit
not
demonstrated with durvalumab
monotherapy
†
in patients with
PD-L1
≥25%
Cross-study comparisons are not intended.
*For primary analysis. Patients with ≥1% PD-L1 expression were eligible.
†
Not yet formally tested.
1. Reck M et al.
N Engl J Med
. 2016;375(19):1823-1833. 2. Lopes G et al. Oral presentation at ASCO 2018. 105. 3. Carbone DP et al.
N Engl J Med
. 2017;376(25):2415-2426. 4.
Peters S et al. Poster presentation at ELCC 2016. 191TiP. 5. AstraZeneca [press release]. July 27, 2017.
MSD
AZ
AZ
BMS








