Langer CJ, et al. Lancet Oncol 17:1497-1508, 2016;
Borghaei H, et al. J Thorac Oncol [Epub ahead of print], 2018
First-line CT
±
Pembrolizumab for advanced non-squamous NSCLC
Randomized phase II
cohort of open-label multicohort trial
Pts with stage IIIB/IV
nonsquamous
NSCLC and
ECOG PS 0/1, no previous
systemic therapy
, no
actionable
EGFR/ALK
mutations
(N = 123)
Pembrolizumab 200 mg IV
+ Cb/Pem* Q3W x 4
(n = 60)
Cb/Pem* Q3W x 4
(n = 63)
Stratified by PD-L1 TPS (< 1% vs ≥ 1%)
Pembrolizumab up to 24 mos
+ Pemetrexed maintenance
†
Pemetrexed maintenance
†
*Cb AUC 5 mg/mL/min; pembro 500 mg/m
2
.
†
Optional.
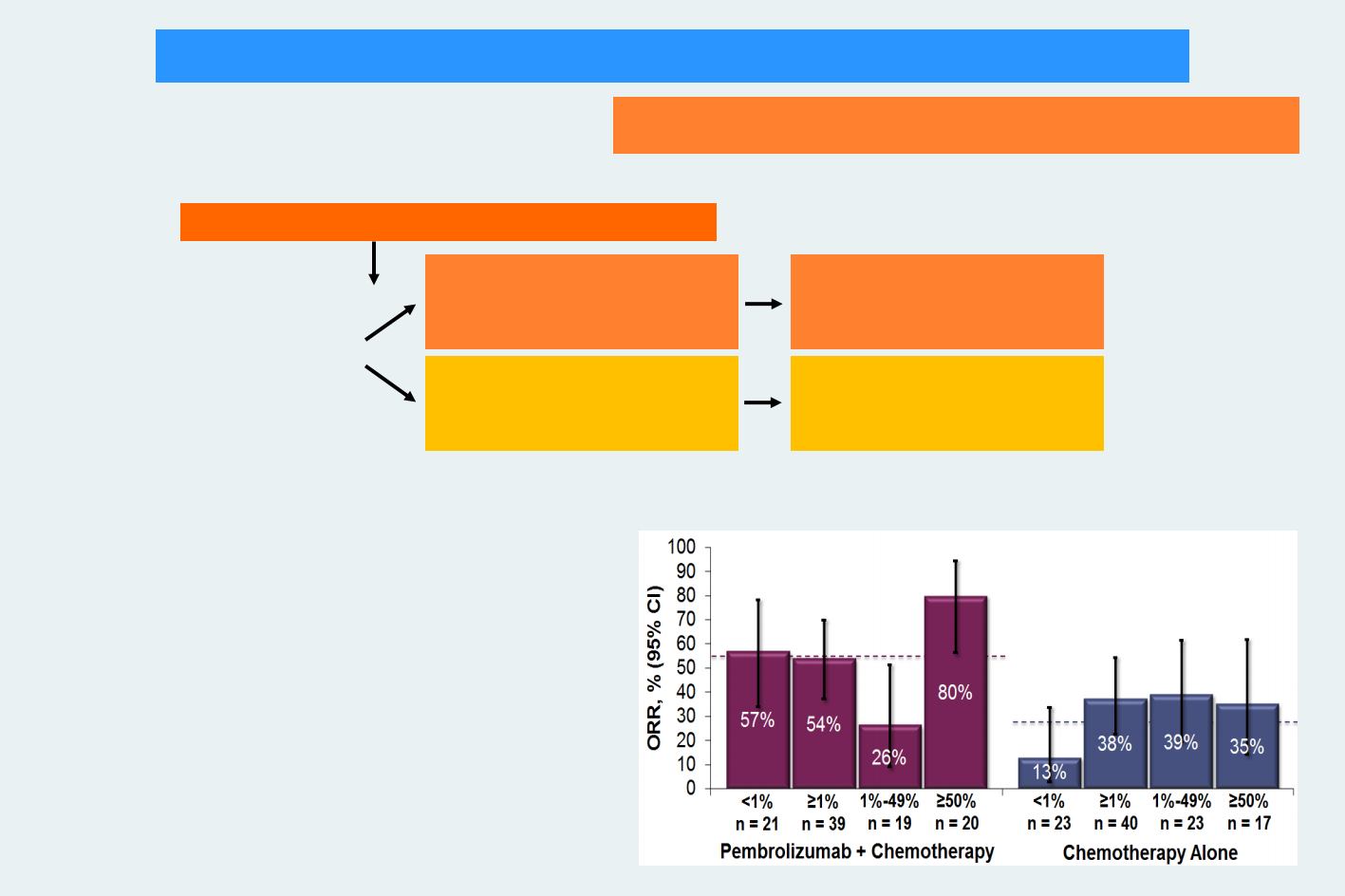
Primary endpoint: ORR (RECIST v1.1)
Secondary endpoints: PFS, DoR, OS, and safety
KEYNOTE-021 G trial








