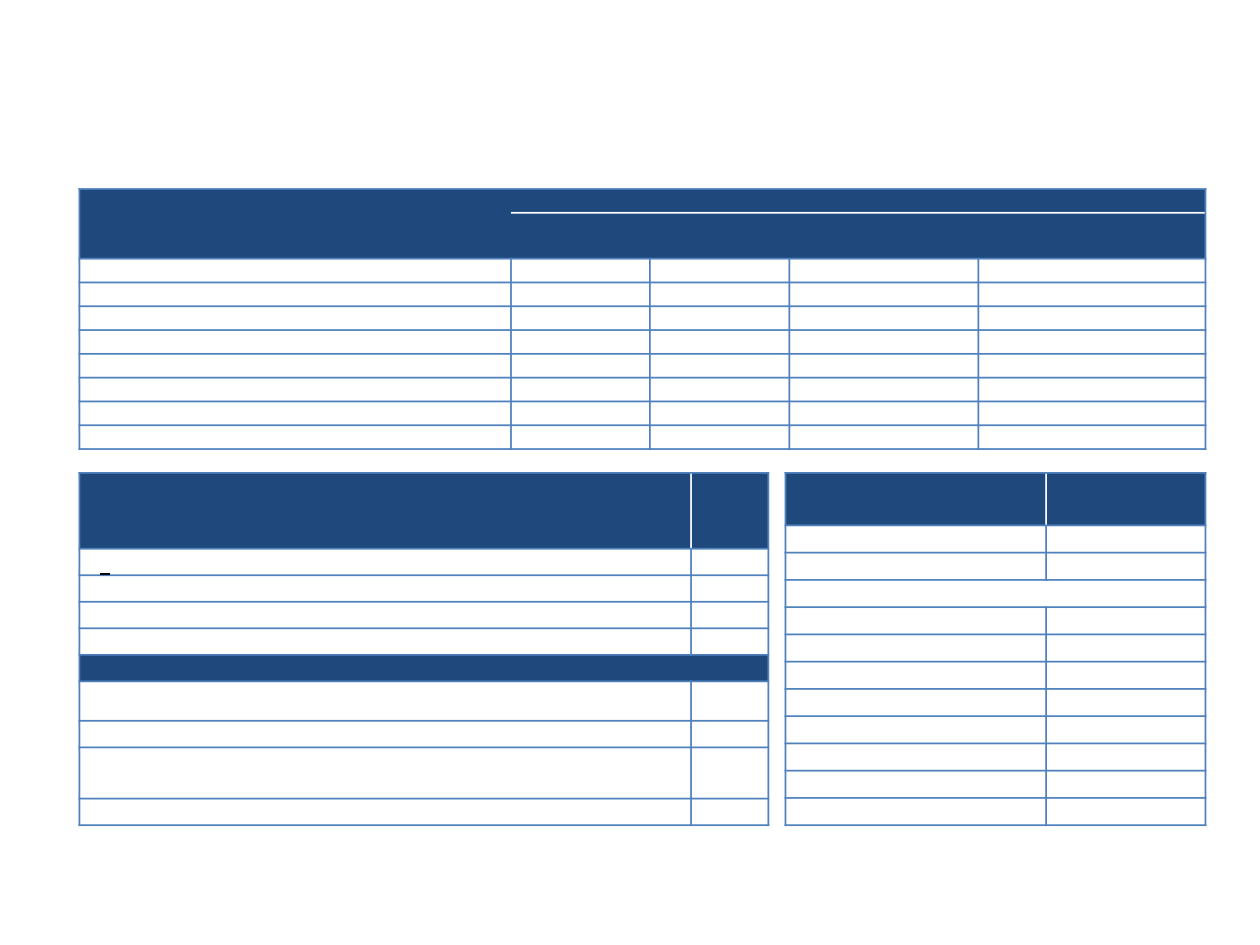
MSKCC
NAPOLI-1
1
nal-IRI+5-FU/LV
(n = 56)
nal-IRI+5-FU/LV
(n = 117)
Toxicities
Any grade (%) Grade 3/4 (%)
Any grade (%)
Grade 3/4 (%)
Nausea
33 (59)
2 (4)
60 (51)
9 (8)
Vomiting
18 (32)
2 (4)
61 (52)
13 (11)
Diarrhoea
35 (63)
1 (2)
69 (59)
15 (13)
Fatigue
45 (80)
1 (2)
47 (40)
16 (14)
Anorexia
32 (57)
0 (0)
52 (44)
5 (4)
Neutropenia
16 (29)
1 (2)
46 (39)
32 (27)
Anaemia
50 (89)
10 (18)
44 (38)
11 (9)
Single centre experience: AEs, dosing, dose reductions and
treatment sequencing
AE, Adverse event; MSKCC, Memorial Sloan-Kettering Cancer
centre
Glassman DC, et al. J Clin Oncol 2018;
36 (suppl 4S): 471 (and poster)
Dose reductions
n = 18
n (%)
1
15 (83)
2
3 (17)
Reason attributed for dose reduction
Fatigue
8 (44)
Diarrhea
8 (44)
Nausea
2 (11)
Neutropenia
2 (11)
Anorexia
2 (11)
Abdominal cramping
1 (6)
Ageusia
1 (6)
Not defined
1 (6)
Starting dose
nal-IRI (mg/m
2
)
n = 56
%
≤50
23 (41)
55
9 (16)
60
7 (13)
70
17 (30)
Treatment sequencing
FOLF(IRIN)OX ↔ gemcitabine+(nab-paclitaxel)
à
nal-IRI+5-FU/LV
26 (46)
Gemcitabine+nab-paclitaxel
à
nal-IRI+5-FU/LV
25 (45)
Gemcitabine+nab-paclitaxel
à
Gemcitabine/capecitabine
à
nal-IRI+5-FU/LV
3 (5)
Other
2 (4)








