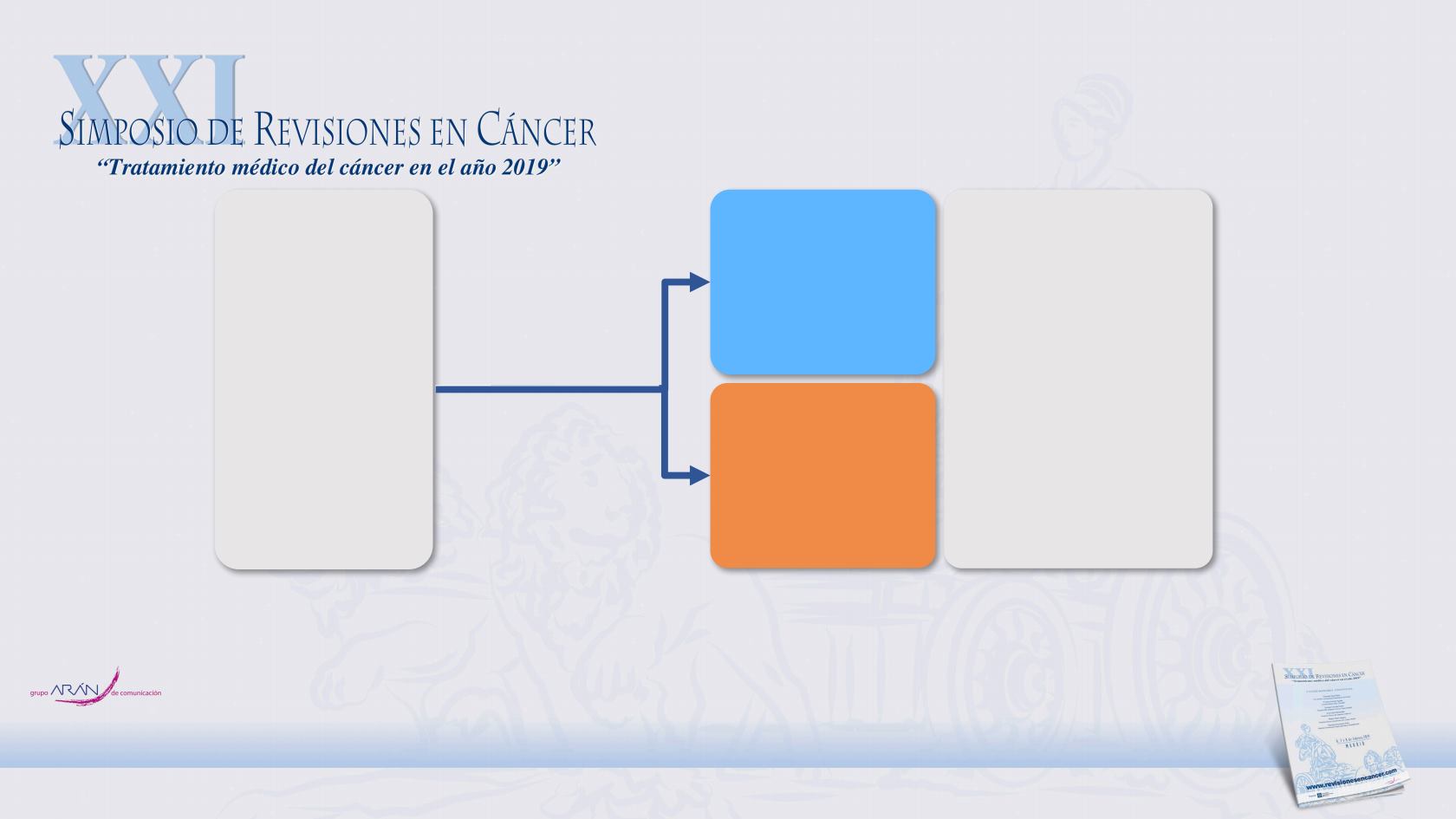
MONALEESA-3: Phase III placebo-controlled study of
ribociclib + fulvestrant
8
•
Tumor assessments were performed every 8 weeks for 18 months, then every 12 weeks thereafter
•
Primary analysis planned after ~364 PFS events
•
95% power to detect a 33% risk reduction (hazard ratio 0.67) with one-sided α=2.5%, corresponding to an increase in median PFS to 13.4 months
(median PFS of 9 months for the placebo arm), and a sample size of 660 patients
ECOG PS, Eastern Cooperative Oncology Group performance status; RECIST, Response Evaluation Criteria In Solid Tumors.
*Fulvestrant administered intramuscularly on Cycle 1 Day 1, Cycle 1 Day 15, and Day 1 of every 28-day cycle thereafter.
Stratified by:
•
Presence/absence of
liver/lung metastases
•
Prior endocrine therapy
Primary endpoint
•
PFS (locally assessed per
RECIST v1.1)
Secondary endpoints
•
Overall survival
•
Overall response rate
•
Clinical benefit rate
•
Time to response
•
Duration of response
•
Time to definitive
deterioration
of ECOG PS
•
Patient-reported outcomes
•
Safety
•
Pharmacokinetics
•
Postmenopausal
women and men
with HR+/HER2–
ABC
•
No or ≤1 line of
prior endocrine
therapy for
advanced disease
•
N=726
Randomization
(2:1)
Ribociclib
(600 mg/day orally;
3-weeks-on/1-week-off)
+
fulvestrant
(500 mg)*
n=484
Placebo
+
fulvestrant
(500 mg)*
n=242








