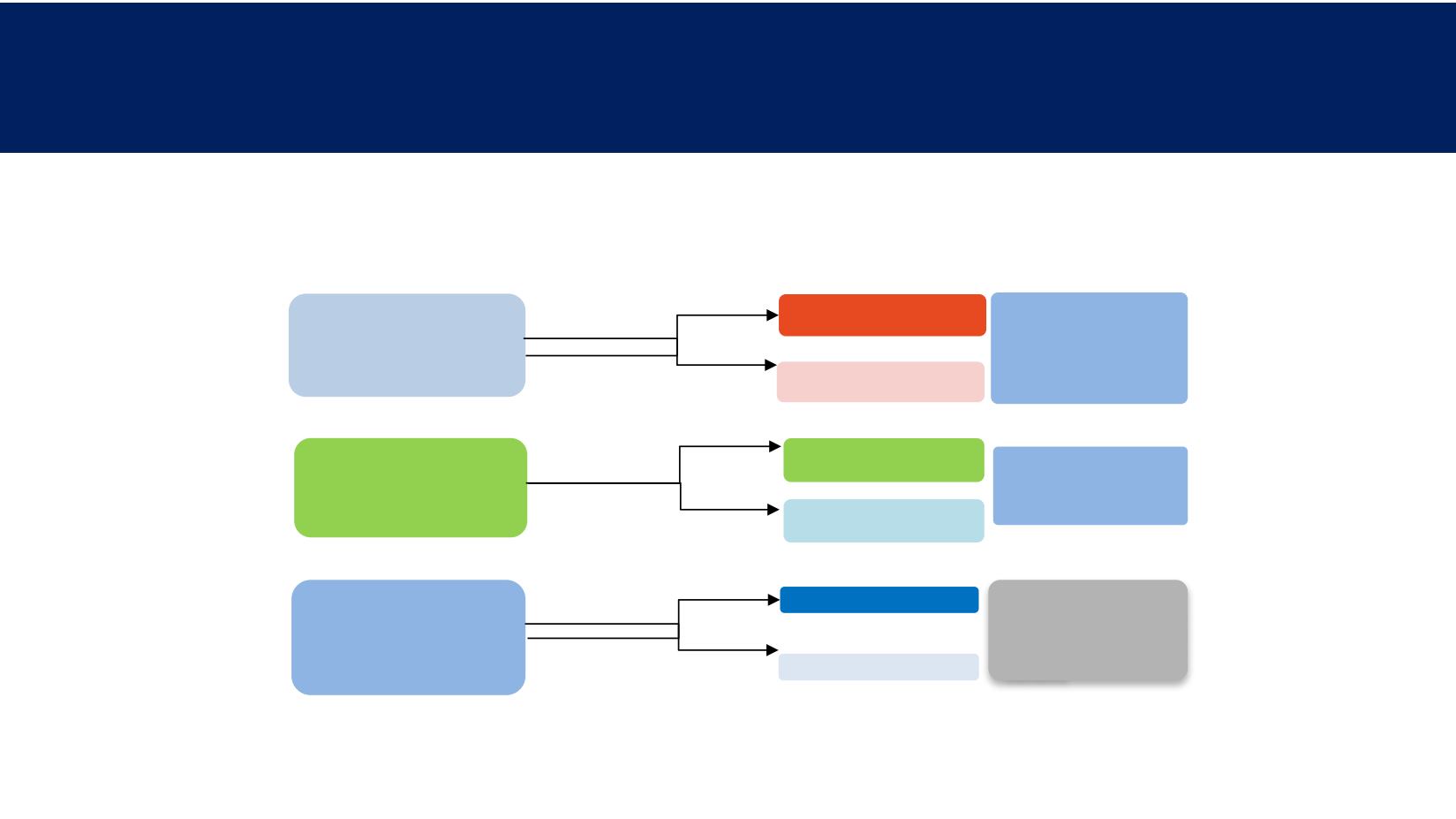
Estudios Fase III de Registro
Palbociclib + Letrozole
n = 444
Placebo + Letrozole
n = 222
Postmenopausal women with HR+,
HER2– advanced breast cancer (N =
666)
No prior therapy for
advanced disease
Randomization (2:1)
Primary endpoint
PFS
Secondary endpoints
OS, ORR, safety, PRO
Primary endpoint
PFS
Secondary endpoints
OS, DoR, DCR, CBR,
ORR, PRO, safety,
tolerability
Abemaciclib + NSAI
Postmenopausal women with
HR+, HER2– advanced breast
cancer (N = 450)
No prior therapy for
advanced disease
Randomization (2:1)
Placebo + NSAI
Ribociclib + Letrozole
n = 334
Placebo + Letrozole
n = 334
Primary endpoint
PFS
Secondary endpoints
OS (key), ORR, CBR,
Safety, PRO, TTD for ECOG PS
Postmenopausal women with
HR+, HER2– advanced breast
cancer (N = 668)
No prior therapy for advanced
disease
Randomization (1:1)
MONALEESA-2
PALOMA-2
MONARCH-3
Nuevas Combinaciones con Inhibidores del ciclo celular








