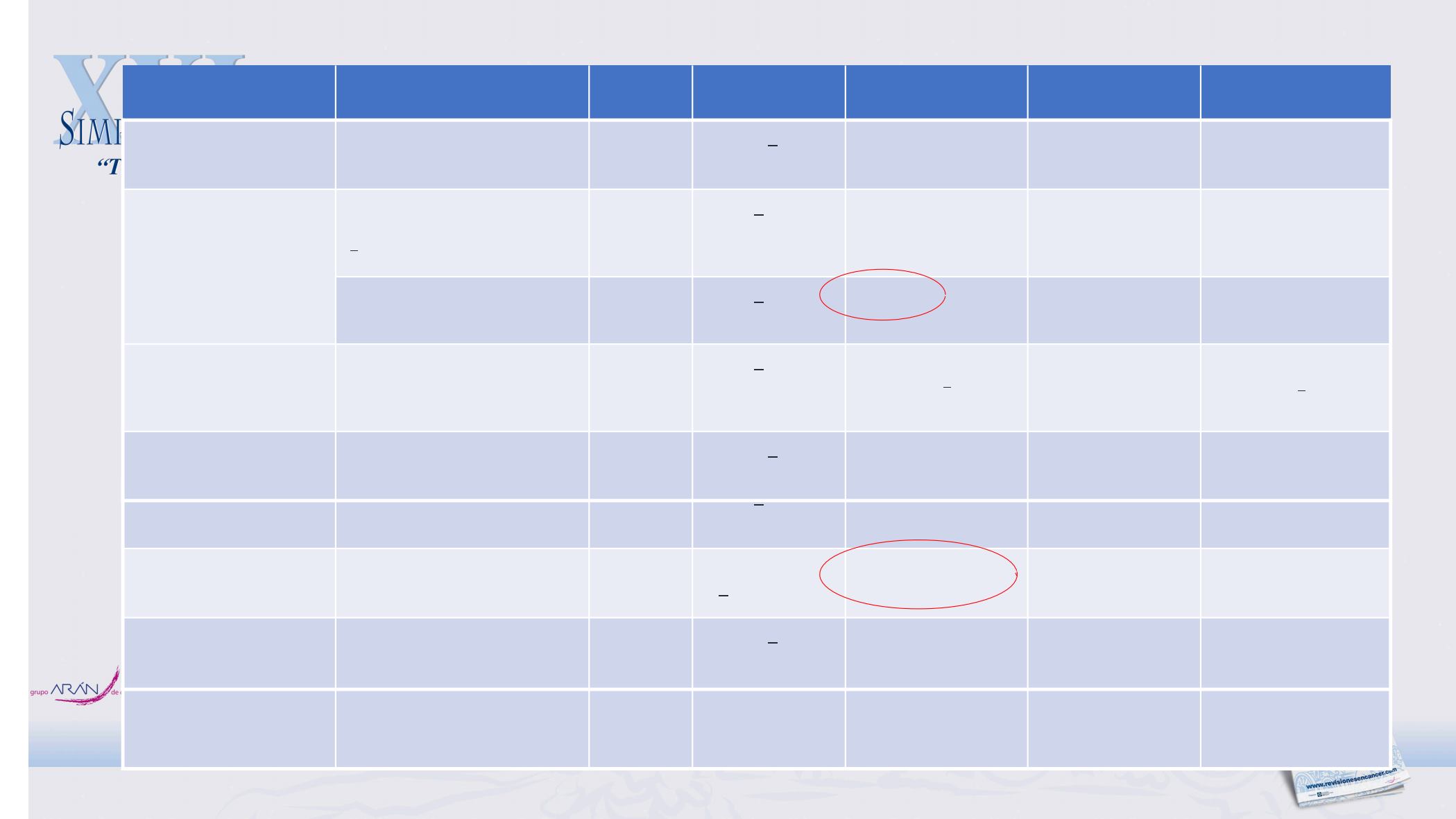
Study
Design
Population
N
SAFETY
ORR (%)
PFS (mo)
OS (mo)
ITT2017-07-HT-
PembroRT
McArthur et al 2018
II Pembro+RT
mTNBC & PD-L1+/-
1L-3L
17
Mild AEs
18
2/9 evaluable
NA
NA
Study
Design
Population
N
SAFETY
ORR (%)
PFS (mo)
OS (mo)
GP28328
Adams et al 2018
I Atezo+NabP
mTNBC & PD-L1+/-
39% 1L
33
73% G>3
39.4
54% 1L
5.5
8.6 1L
14.7
24 1L
IMpassion-130
Schmid et al 2018
III Atezo+NabP
mTNBC & PD-L1+/-
1L
451
48.7 vs 42.2%
G>3
45.9 vs 56
42.6 vs 58.9 PD-L1+
5.5 vs 7.2
5 vs 7.5 PD-L1+
17.6 vs 21.3 ITT
15.5 vs 25 PD-L1+
ENHANCE
Tolaney et al 2018
Ib/II Pembro+Eribul
mTNBC & PD-L1+/-
1L-3L
107
66.4% G>3
26
29.2% 1L
4.2
4.9 1L
17.7
17.7 1L
Vonderheide et al CCR 2017; Adams S SABCS 2018
Study
Design
Population
N
SAFETY
ORR (%)
PFS (mo)
OS (mo)
KEYNOTE-012
Nanda et al 2016
Ib Pembrolizumab
mTNBC & PD-L1+
15.6% 1L
32
15.6% G>3
18.5
1.9
11.2
KEYNOTE-086
Adams et al 2017 & 2018
II Pembrolizumab
mTNBC & PD-L1+/-
>2L
170
12% G>3
4.7
2
8.9
II Pembrolizumab
mTNBC & PD-L1+
1L
84
10% G>3
21.4
2.1
18.0
PCD4989g
Emens et al 2018
Ia Atezolizumab
mTNBC & PD-L1+later expanded
17% 1L
116
11% G>3
10
24% 1L & 6% >2L
12% PD-L1+
1.4
9.3
1
7.6 1L & 7.6>2L
10.1 PD-L1+
JAVELIN
Dirix et al 2018
Ib Avelumab
mBC & PD-L1+/-
1L-4L
58
TNBC
13.7% G>3
5.2 TNBC
NA
NA
ICI monotherapy
ICI+CT combos








