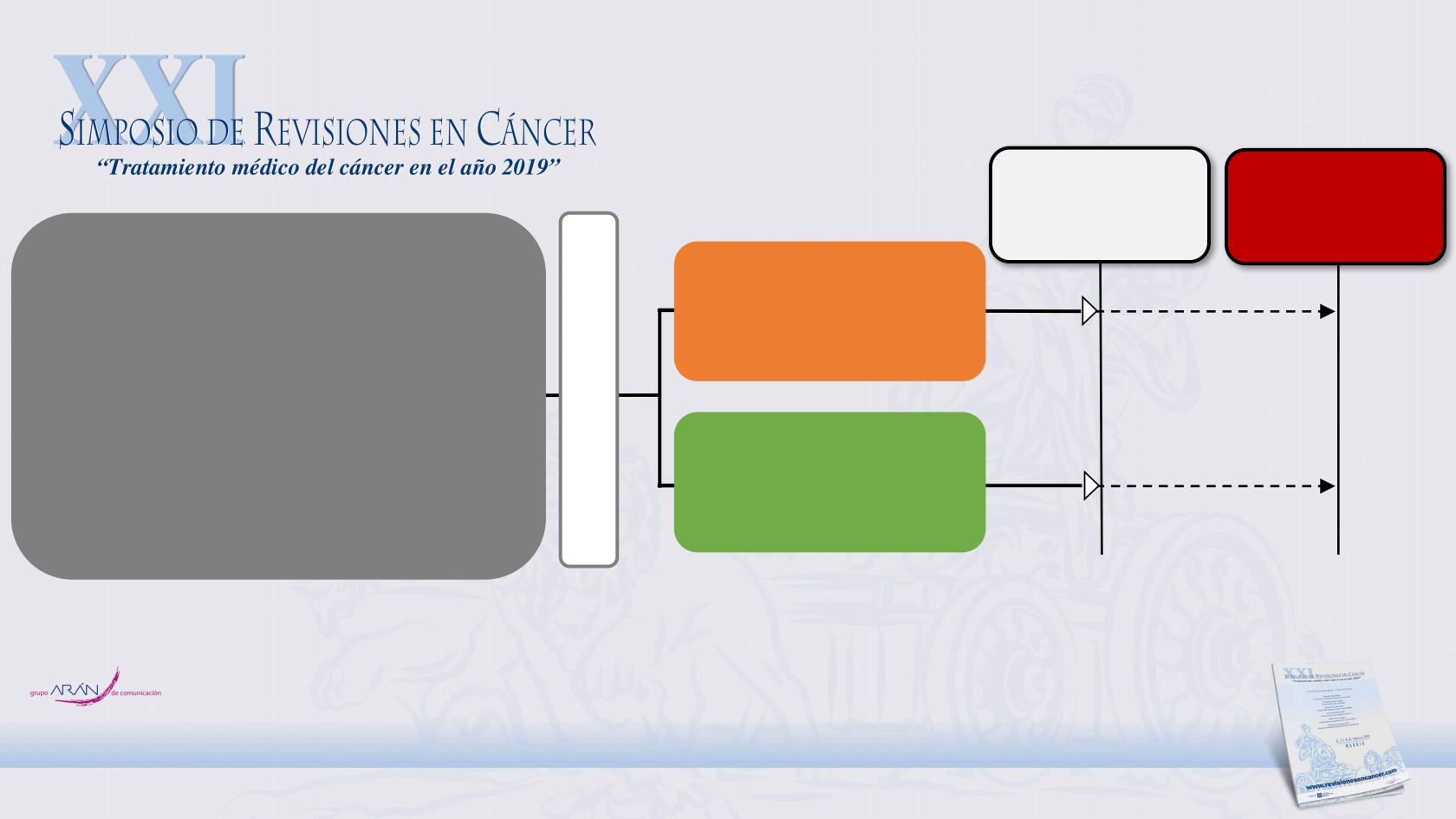
COMBI-AD: STUDY DESIGN
BID, twice daily; DMFS, distant metastasis–free survival; D+T, dabrafenib + trametinib; ECOG, Eastern Cooperative Oncology Group; FFR, freedom
from relapse; FU, follow-up; QD, once daily.
Long GV, et al.
N Engl J Med
. 2017;377:1813-1823.
Key eligibility criteria
•
Completely resected
stage IIIA
(lymph node
metastasis > 1 mm),
IIIB, or IIIC
cutaneous
melanoma
•
BRAF
V600E/K
mutation
•
ECOG
performance status
0 or 1
•
No prior radiotherapy or systemic therapy
•
Tissue collection was mandatory at baseline
and optional upon recurrence
R
A
N
D
O
M
I
Z
A
T
I
O
N
Stratification
•
BRAF
mutation status (V600E, V600K)
•
Disease stage (IIIA, IIIB, IIIC)
1:1
•
Primary endpoint:
RFS
•
Secondary endpoints: OS, DMFS,
FFR, safety
N = 870
Treatment duration:
12 months
Primary analysis
D+T median FU,
33 months
Updated analysis
D+T median FU,
44 months
Dabrafenib 150 mg BID
+ trametinib 2 mg QD
(n = 438)
2 matched placebos
(n = 432)
Long G, et al. Engl J Med 2017;377:1813-23
Hauschild A, et al. J Clin Oncol 2018 Oct 22








