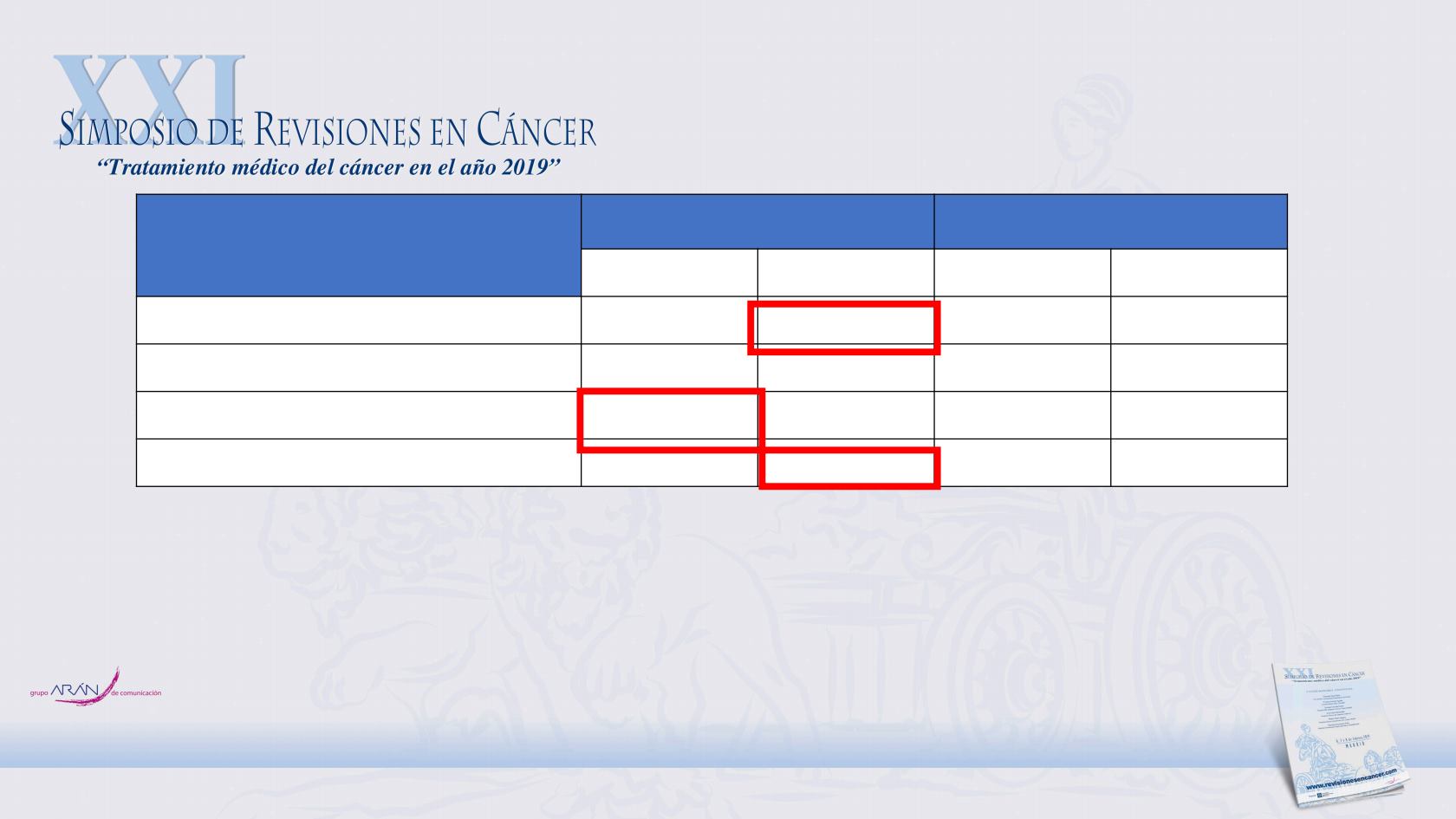
SEGURIDAD
Ipilimumab
(n = 471)
Placebo
(n = 474)
Any Grade
Grade 3/4
Any grade
Grade 3/4
Any AE, %
98.7
54.1
91.1
26.2
Treatment-related AE, %
94.1
45.4
59.9
4.0
Treatment-related AE leading to discontinuation,
%
48.0
32.9
1.5
0.6
Any immune-related AE, %
90.4
41.6
39.7
2.7
• No new deaths due to drug-related AEs compared with the primary analysis
o
5 patients (1.1%) in the ipilimumab group
§
3 patients with colitis (2 with gastrointestinal perforations)
§
1 patient with myocarditis
§
1 patient had multiorgan failure with Guillain-Barré syndrome
o
No deaths related to study drug in the placebo group
Eggermont AM et al. Lancet Oncol 2015;16:522-30








