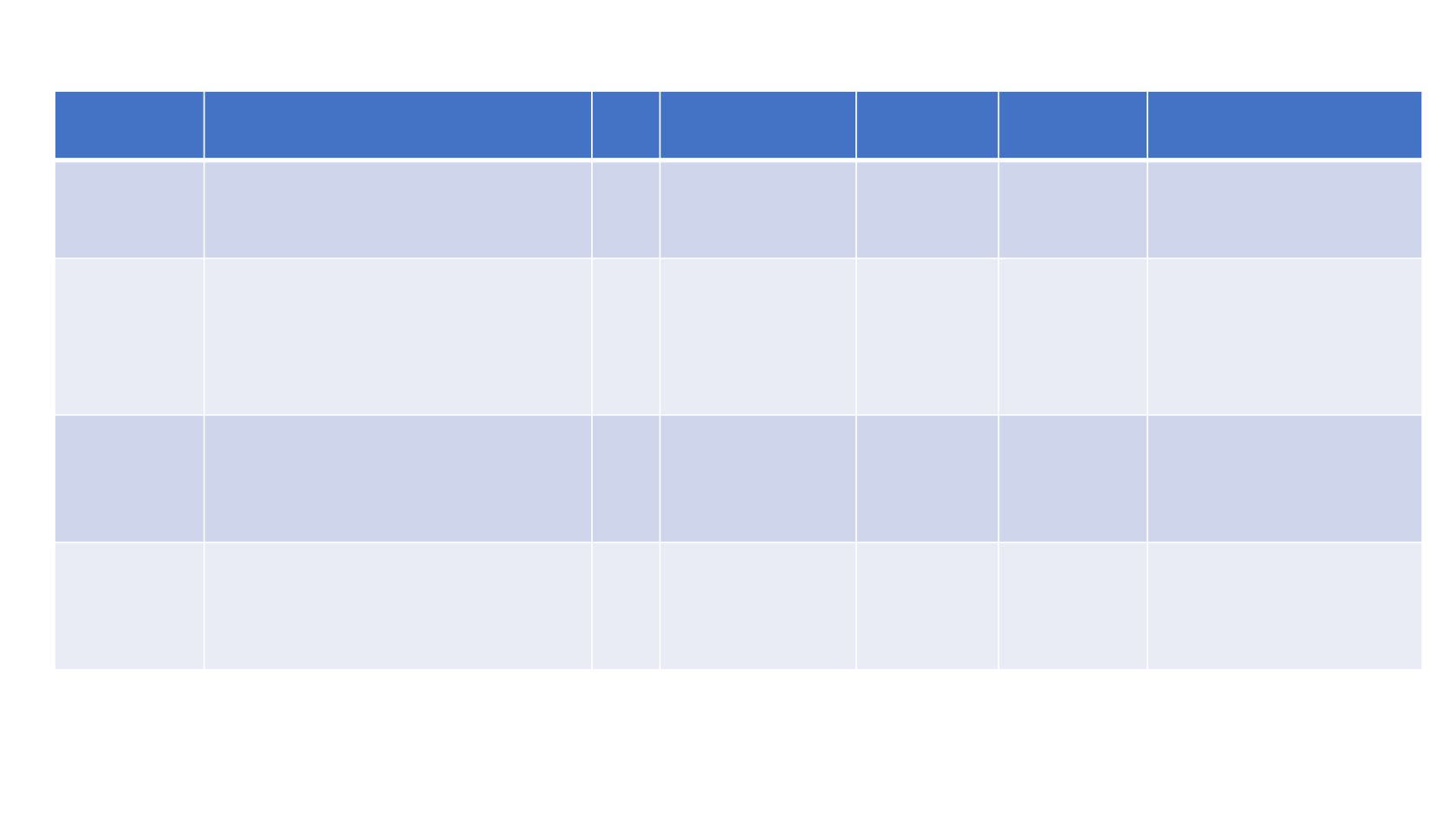
PRRT COMBINATIONS
1.- EJNMMI 2011
2.- Cancer Biother Radiopharm 2012
3.- Cancer Biother Radiopharm 2015
4.- EJNMMI 2015
Author
Treatment
N Response Rate
PFS
OS
Toxicity
Claringbold
1 177
Lu 7.8 GBq x 4
Capecitabine 1650 mg/m
2
/d x 14
33
PR 24%
SD 70%
NR
1 year 91%
2 years 88%
Thrombocytopenia G3
(1p)
Claringbold
2 177
Lu 7.8 GBq x 4
Capecitabine 1650 mg/m
2
/d x 14
TMZ 100-150-200 mg/m
2
x 5
35
CR 15%
PR 38%
SD 38%
31 months 2 years 94% Nausea G2 (18%) G3 (3%)
Thrombocytopenia G2
(24%)
Neutropenia G3 (6%)
Claringbold
3 177
Lu 7.8 GBq x 4
Everolimus 5mg/7.5 mg/10 mg
16
CR 15%
PR 38%
SD 38%
-------------- -------------
MTD 7.5 mg/day
Kashyap
4
177
Lu 6-10 GBq x 3-5 - Maintenance
5Fluorouracil 200 mg/m
2
CI x 3
weeks from 2
nd
cycle
52
PR 28%
SD 68%
48 months
Thrombocytopenia G3/4
(6%)
Hepatotoxicity G3 (1p)








