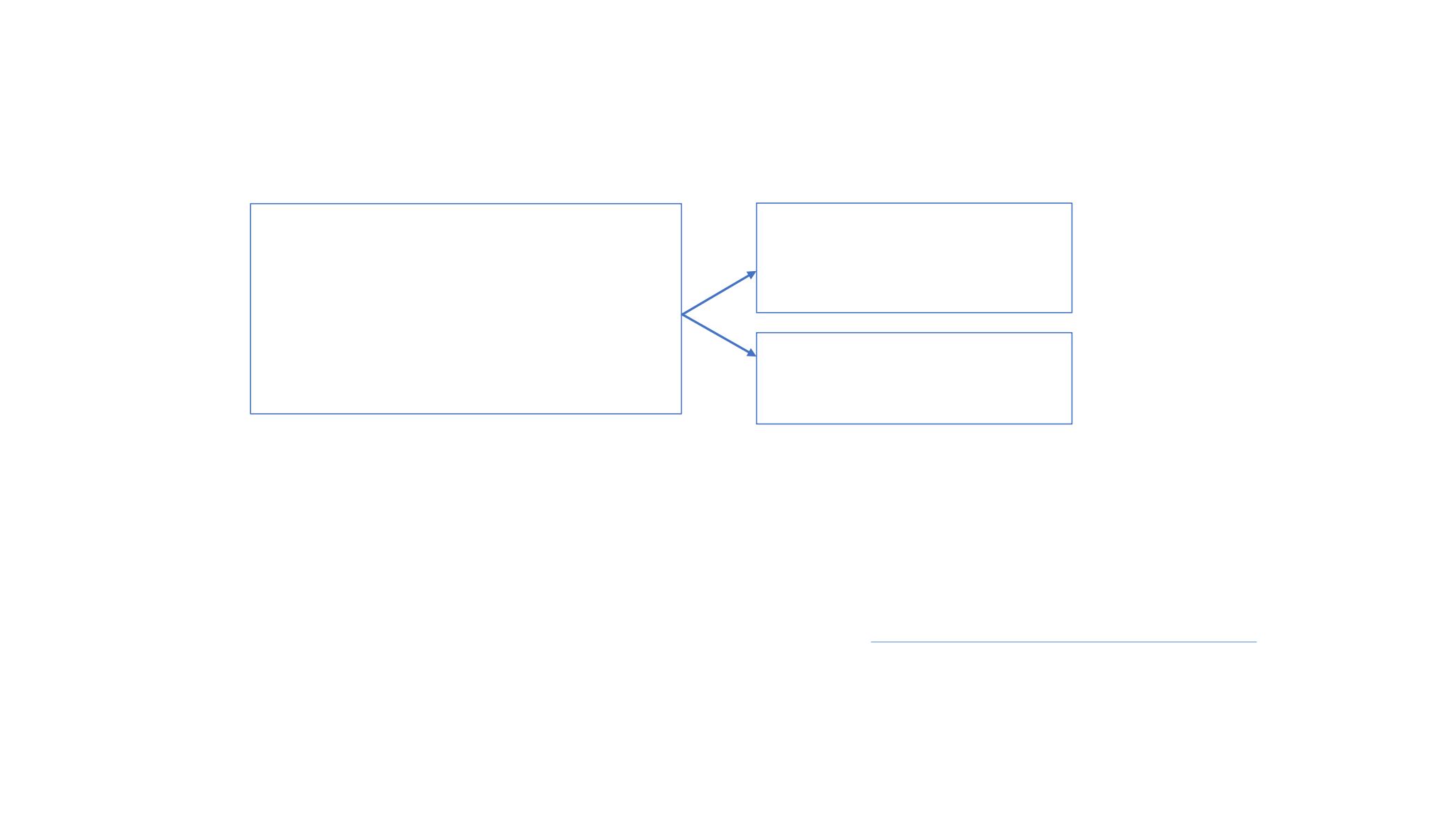
AIO-STO-0415: RAMIRIS trial
25
•
Gastric/GEJ adenocarcinoma
•
PD during or within 6m of the last dose
of 1L platinum and fluoropyrimidine
doublet with or without anthracycline
or docetaxel
•
PS0-1
N=111
https://clinicaltrials.gov/ct2/show/NCT03081143?term=RAMIRIS&rank=1FOLFIRI
+
Ramucirumab
28-day cycle
Paclitaxel
+
Ramucirumab
28-day cycle
Primary endpoint
•
OS at 6 months
Secondary endpoints
•
PFS
•
ORR
•
DCR
•
Safety
•
QOL
PI: Sylvie Lorenzen, MD
Trial contact person: Salah-Eddin Al-Batran, Prof








